Home /
Expert Answers /
Chemistry /
given-the-following-equilibrium-constants-at-437-deg-c-na-2-o-s-2na-l-1-2-o-2-g-k-1-4-pa433
(Solved): Given the following equilibrium constants at 437\deg C, Na_(2)O(s)2Na(l)+(1)/(2)O_(2)(g),K_(1)=4\ ...
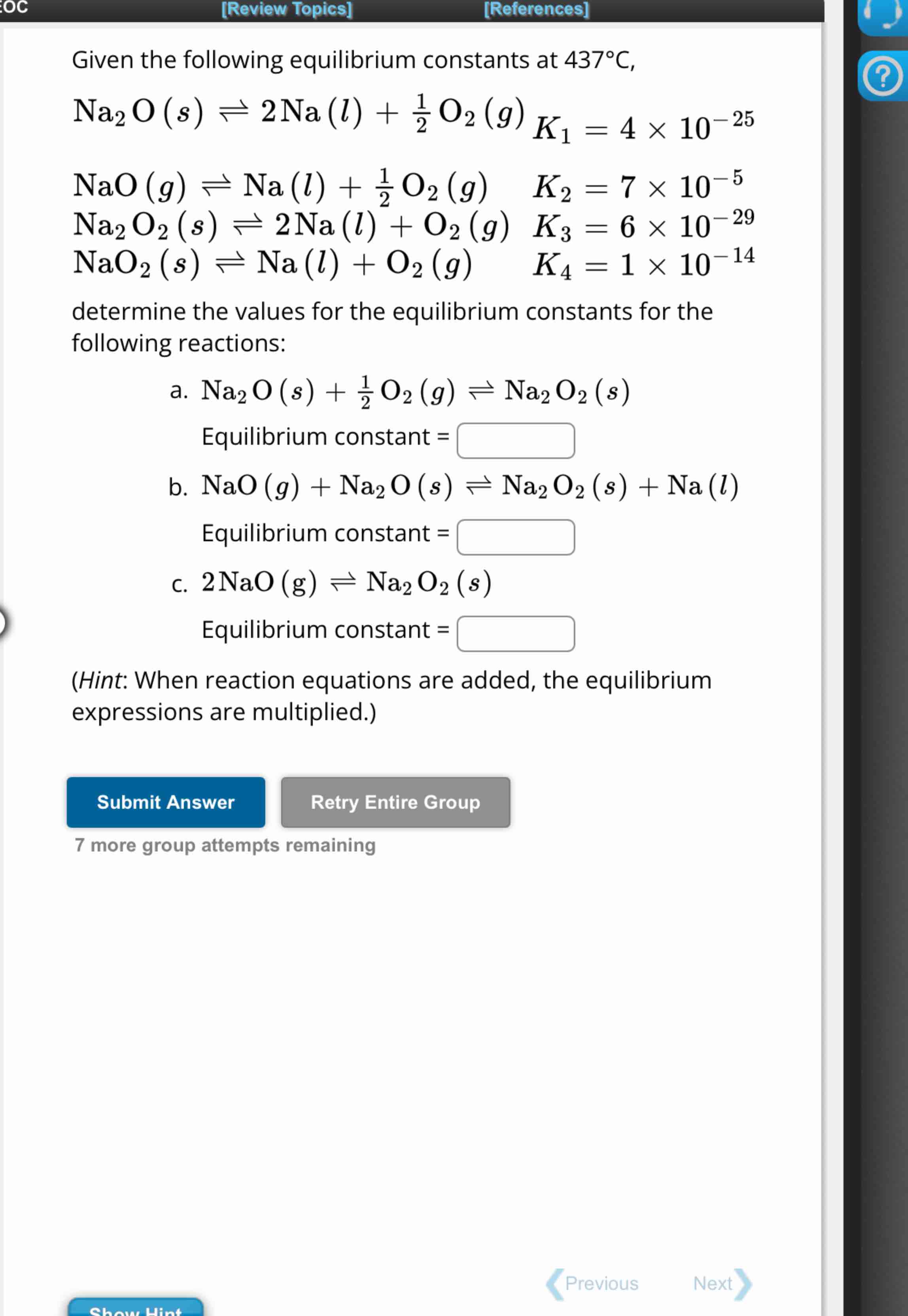
Given the following equilibrium constants at 437\deg C,
Na_(2)O(s)?2Na(l)+(1)/(2)O_(2)(g),K_(1)=4\times 10^(-25)
,
NaO(g)?Na(l)+(1)/(2)O_(2)(g),K_(2)=7\times 10^(-5)
Na_(2)O_(2)(s)?2Na(l)+O_(2)(g),K_(3)=6\times 10^(-29)
NaO_(2)(s)?Na(l)+O_(2)(g),K_(4)=1\times 10^(-14)
determine the values for the equilibrium constants for the
following reactions:
a. Na_(2)O(s)+(1)/(2)O_(2)(g)?Na_(2)O_(2)(s)
Equilibrium constant =
b. NaO(g)+Na_(2)O(s)?Na_(2)O_(2)(s)+Na(l)
Equilibrium constant =
c. 2NaO(g)?Na_(2)O_(2)(s)
Equilibrium constant =
(Hint: When reaction equations are added, the equilibrium
expressions are multiplied.)
7 more group attempts remaining