Home /
Expert Answers /
Chemistry /
help-needed-urgently-thank-you-in-advance-the-ph-of-0-45m-of-an-uniknown-base-is-10-87-the-pk6-pa248
(Solved): help needed urgently, thank you in advance! The pH of 0.45M of an uniknown base is 10.87 . The pK6 ...
help needed urgently, thank you in advance!
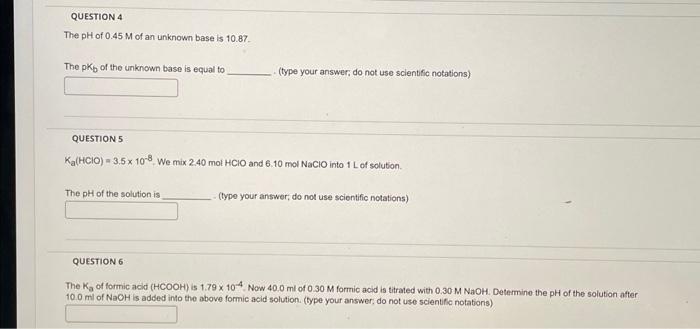

The of of an uniknown base is 10.87 . The of the unknown base is equal to . (type your answer, do not use scientific notations) QUESTION 5 . We mix and into of solution. The of the solution is - (type your answer; do not use scientific notations) QUESTION 6 The of formic acld is of formic acid is titrated with . Determine the pH of the solution after of is added into the above formic acid solution. (type your answer, do not use scientifo notations)