Home /
Expert Answers /
Chemistry /
helpppp-fat-has-9-calories-per-gram-carbohydrate-has-4-calories-per-gram-and-protein-has-4-calories-pa921
(Solved): helpppp Fat has 9 Calories per gram, carbohydrate has 4 Calories per gram and protein has 4 Calories ...
helpppp
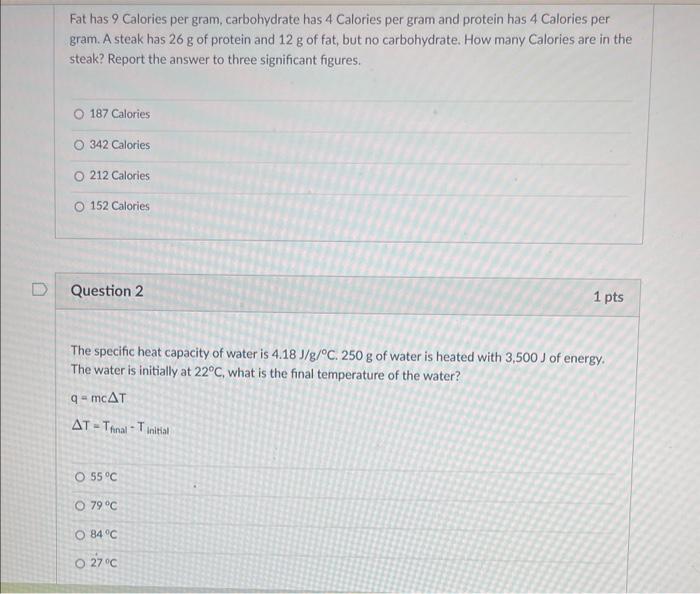



Fat has 9 Calories per gram, carbohydrate has 4 Calories per gram and protein has 4 Calories per gram. A steak has \( 26 \mathrm{~g} \) of protein and \( 12 \mathrm{~g} \) of fat, but no carbohydrate. How many Calories are in the steak? Report the answer to three significant figures. 187 Calories 342 Calories 212 Calories 152 Calories Question 2 1 pts The specific heat capacity of water is \( 4.18 \mathrm{~J} / \mathrm{g} /{ }^{\circ} \mathrm{C} .250 \mathrm{~g} \) of water is heated with \( 3,500 \mathrm{~J} \) of energy. The water is initially at \( 22^{\circ} \mathrm{C} \), what is the final temperature of the water? \[ q=m c \Delta T \] \( \Delta T=T_{\text {final }}-T_{\text {initisl }} \) \( 55^{\circ} \mathrm{C} \) \( 79^{\circ} \mathrm{C} \) \( 84^{\circ} \mathrm{C} \) \( 27^{\circ} \mathrm{C} \)
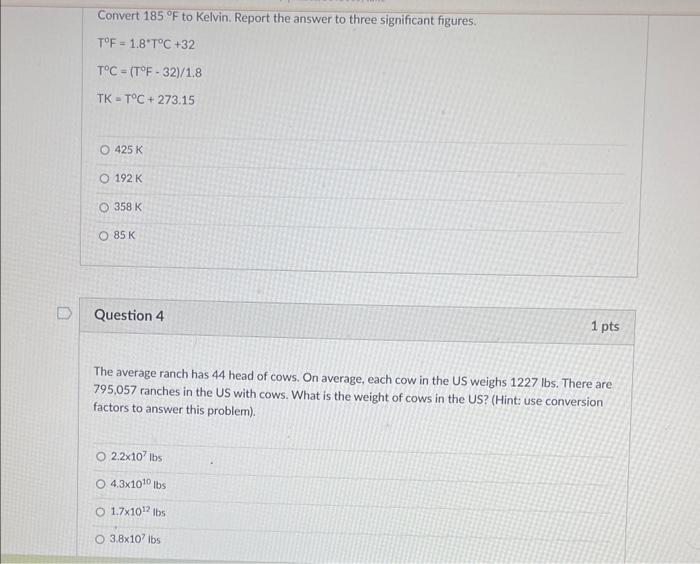
Convert \( 185^{\circ} \mathrm{F} \) to Kelvin. Report the answer to three significant figures. \[ \begin{array}{l} T^{\circ} \mathrm{F}=1.8^{\circ} \mathrm{T}^{\circ} \mathrm{C}+32 \\ T^{\circ} \mathrm{C}=\left(\mathrm{T}^{\circ} \mathrm{F}-32\right) / 1.8 \\ T K=T^{\circ} \mathrm{C}+273.15 \end{array} \] \( 425 \mathrm{~K} \) \( 192 \mathrm{~K} \) \( 358 \mathrm{~K} \) \( 85 \mathrm{~K} \) Question 4 1 pts The average ranch has 44 head of cows. On average, each cow in the US weighs \( 1227 \mathrm{lbs} \). There are 795,057 ranches in the US with cows. What is the weight of cows in the US? (Hint: use conversion factors to answer this problem). \( 2.2 \times 10^{7} \mathrm{lbs} \) \( 4.3 \times 10^{10} \mathrm{lbs} \) \( 1.7 \times 10^{12} \mathrm{lbs} \) \( 3.8 \times 10^{7} \mathrm{lbs} \)
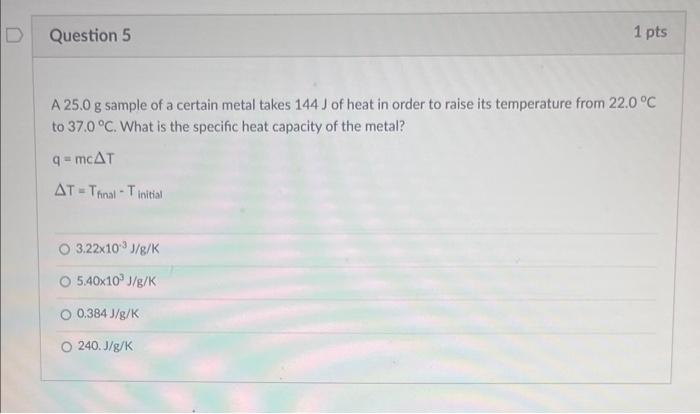
A \( 25.0 \mathrm{~g} \) sample of a certain metal takes \( 144 \mathrm{~J} \) of heat in order to raise its temperature from \( 22.0^{\circ} \mathrm{C} \) to \( 37.0^{\circ} \mathrm{C} \). What is the specific heat capacity of the metal? \[ \begin{array}{l} q=m c \Delta T \\ \Delta T=T_{\text {final }}-T_{\text {initial }} \end{array} \] \( 3.22 \times 10^{-3} \mathrm{~J} / \mathrm{g} / \mathrm{K} \) \( 5.40 \times 10^{3} \mathrm{~J} / \mathrm{g} / \mathrm{K} \) \( 0.384 \mathrm{~J} / \mathrm{g} / \mathrm{K} \) \( 240 . \mathrm{J} / \mathrm{g} / \mathrm{K} \)
Expert Answer
Answer 1) Protein has 4 calories per gram. Generally Fat has 9 calories per gram. A steak has 26 g of protein and 12 g of fat. So total c