Home /
Expert Answers /
Chemistry /
how-did-you-calculate-the-table-data-collection-table-1-addition-of-hcl-to-buffer-and-water-soluti-pa470
(Solved): how did you calculate the table? Data Collection Table 1. Addition of HCl to Buffer and Water Soluti ...
how did you calculate the table?
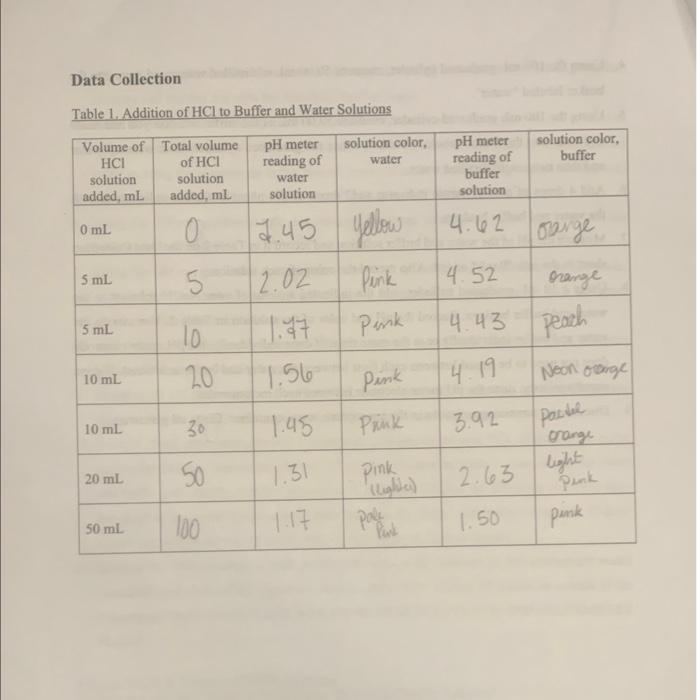




Data Collection Table 1. Addition of HCl to Buffer and Water Solutions
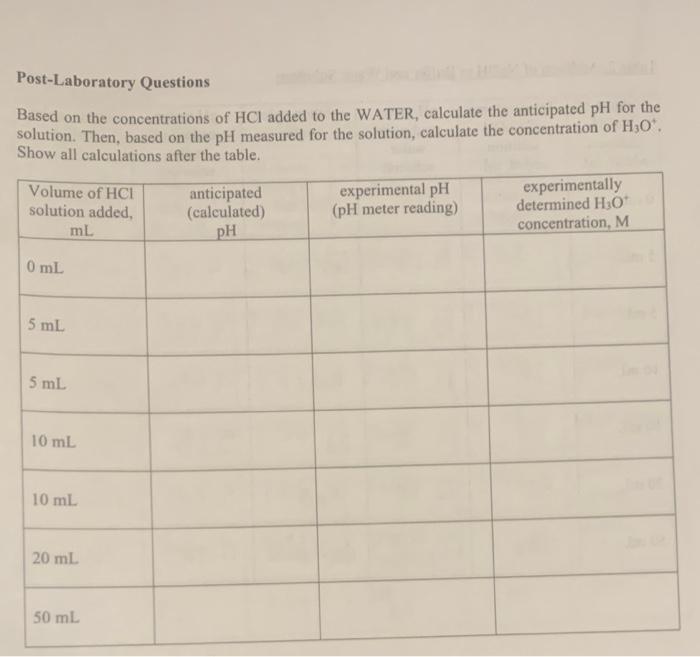
Post-Laboratory Questions Based on the concentrations of \( \mathrm{HCl} \) added to the WATER, calculate the anticipated \( \mathrm{pH} \) for the solution. Then, based on the \( \mathrm{pH} \) measured for the solution, calculate the concentration \( \mathrm{H}_{3} \mathrm{O}^{+} \). Show all calculations after the table.
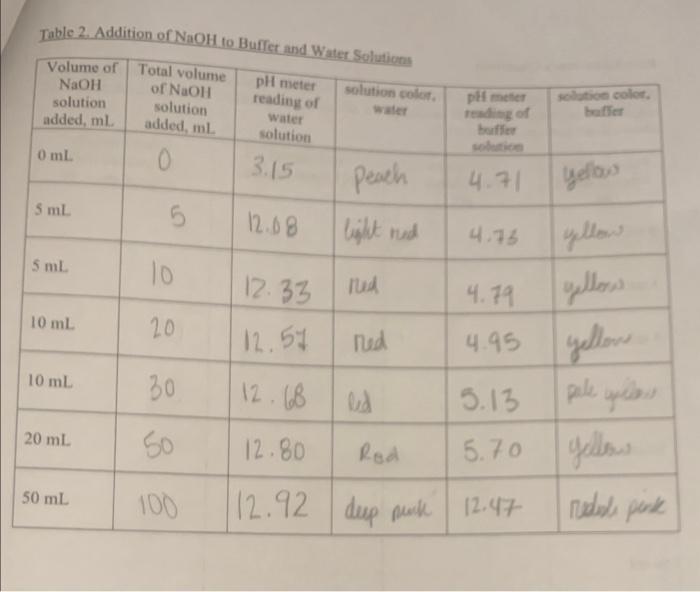
Table 2. Addition of \( \mathrm{NaOH} \) to Buffer and \( \mathrm{W} \)
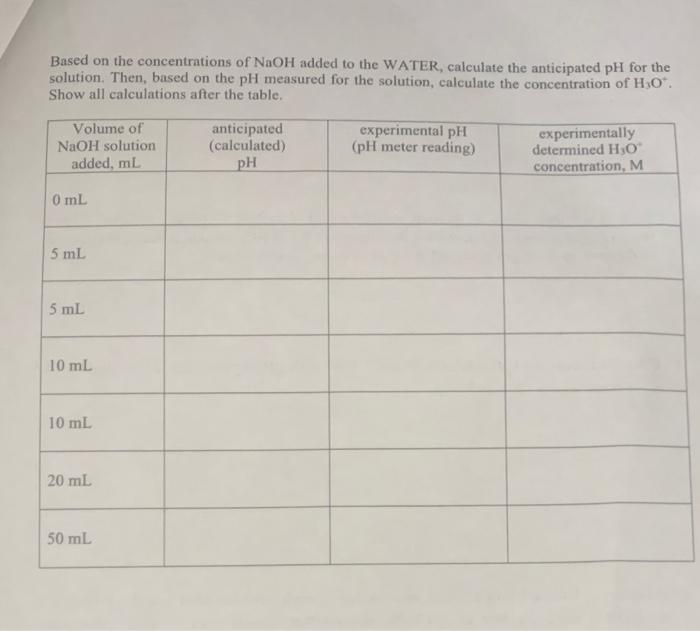
Based on the concentrations of \( \mathrm{NaOH} \) added to the WATER, calculate the anticipated pH for the solution. Then, based on the \( \mathrm{pH} \) measured for the solution, calculate the concentration of \( \mathrm{H}_{3} \mathrm{O}^{+} \). Show all calculations after the table.