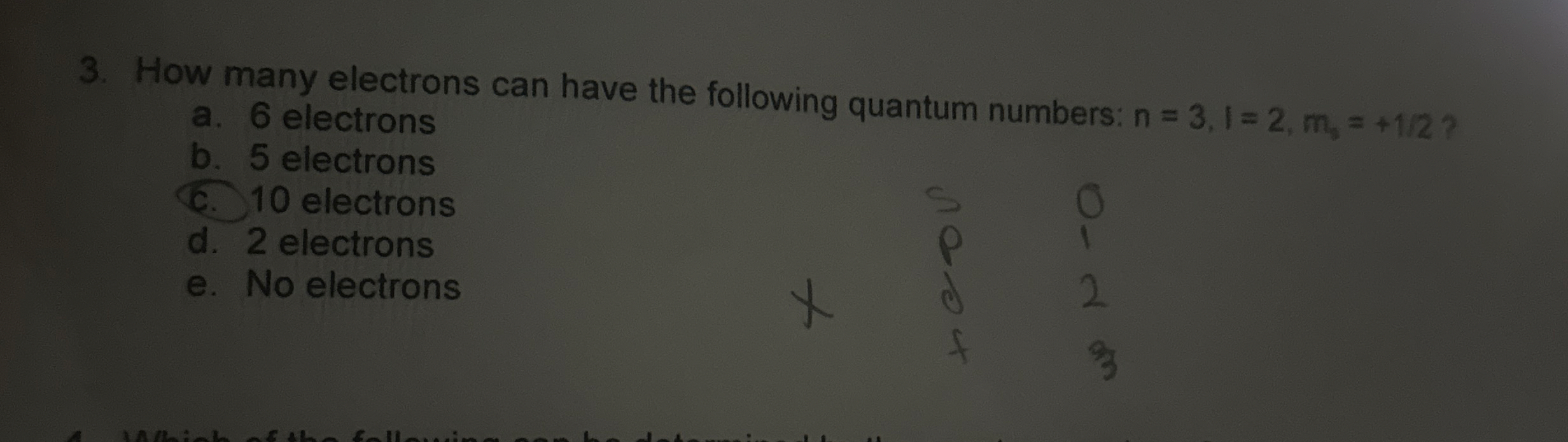Home /
Expert Answers /
Chemistry /
how-many-electrons-can-have-the-following-quantum-numbers-n-3-l-2-m-0-1-2-a-6-electrons-b-pa131
(Solved): How many electrons can have the following quantum numbers: n=3,l=2,m_(0)=+(1)/(2) ? a. 6 electrons b ...
How many electrons can have the following quantum numbers:
n=3,l=2,m_(0)=+(1)/(2)? a. 6 electrons b. 5 electrons C. 10 electrons d. 2 electrons e. No electronsHow many electrons can have the following quantum numbers:
n=3,l=2,m_(0)=+(1)/(2)? a. 6 electrons b. 5 electrons C. 10 electrons d. 2 electrons e. No electrons