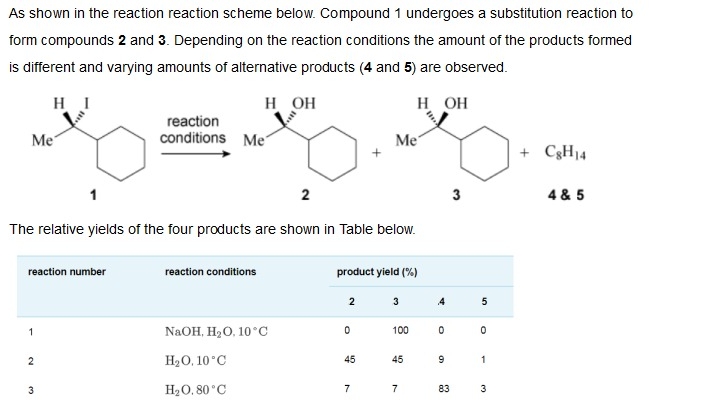
(Solved): (i) Draw the skeletal structures of compounds 4 and 5 and explain why compound 4 is formed in highe ...
(i) Draw the skeletal structures of compounds 4 and 5 and explain why compound 4 is formed in higher yield than compound 5 under reactions conditions 2 and 3. (ii) Based on the product yield information provided, what is the most likely reaction mechanism for the formation of the major products in reactions 1, 2 and 3 and explain briefly on the reason behind. (iii) Draw a curly arrow mechanism, for the formation of product 3 from compound 1 in reaction number 1. Include the transition state of the reaction ensuring the chirality is correct. (iv) How could you adjust the reaction conditions of reaction 3 to further increase the percentage yield of compound 4? As shown in the reaction reaction scheme below. Compound 1 undergoes a substitution reaction to form compounds 2 and 3. Depending on the reaction conditions the amount of the products formed is different and varying amounts of alternative products (
4and 5 ) are observed.
C_(8)H_(14)4& 5 The relative yields of the four products are shown in Table below. \table[[reaction number,reaction conditions,product yield (%)],[,,2,3,4,5],[1,
NaOH,H_(2)O,10\deg C,0,100,0,0],[2,
H_(2)O,10\deg C,45,45,9,1],[3,
H_(2)O,80\deg C,7,7,83,3]]