Home /
Expert Answers /
Chemistry /
in-an-ir-spectra-the-x-axis-is-wavenumber-of-the-ir-radiation-which-is-another-way-to-describe-the-pa734
(Solved): In an IR spectra the X axis is wavenumber of the IR radiation which is another way to describe the _ ...
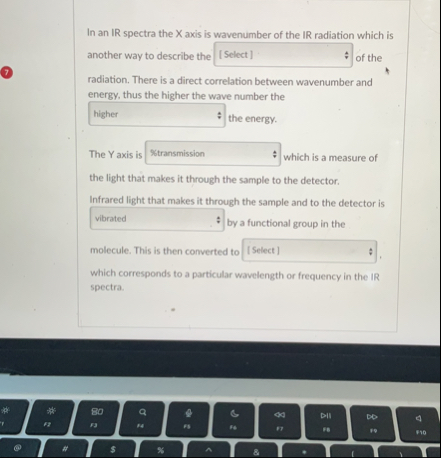
In an IR spectra the X axis is wavenumber of the IR radiation which is another way to describe the
_(_())_(_())of the (7) radiation. There is a direct correlation between wavenumber and energy, thus the higher the wave number the
_(_())_(_())the energy. The Y axis is
_(_())_(_())which is a measure of the ilght that makes it through the sample to the detector. Infrared light that makes it through the sample and to the detector is
_(_())_(_())by a functional group in the molecule. This is then converted to
_(_())_(_())which corresponds to 3 particular wavelength or frequency in the IR spectra.