Home /
Expert Answers /
Chemistry /
in-solution-kmno4-forms-k-ions-and-mno4-ions-permanganate-reacts-with-sodium-oxalate-and-the-endpo-pa629
(Solved): In solution KMnO4 forms K+ ions and MnO4 ions; permanganate reacts with sodium oxalate and the endpo ...
In solution KMnO4 forms K+ ions and MnO4 ions; permanganate reacts with sodium oxalate and the endpoint of the reaction is the appearance of pink solution due to unreacted KMnO4. If 0.1100 g of Na?C?O4 is reacted with 15.56 mL of KMnO4 solution what is the concentration of KMnO4? 2+ 16 H+ + 2 MnO4 +5C?0 ?10 CO? + 2 Mn²+ +8 H?O 2 2- 4
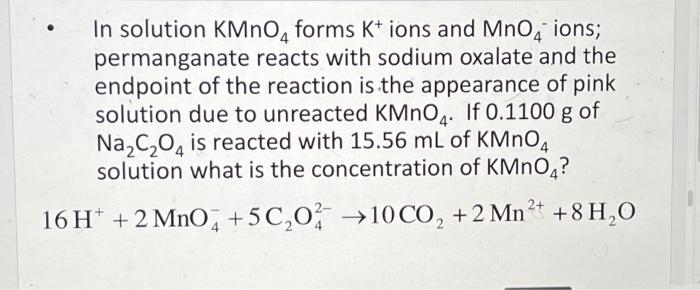
- In solution forms ions and ions; permanganate reacts with sodium oxalate and the endpoint of the reaction is the appearance of pink solution due to unreacted . If of is reacted with of solution what is the concentration of ?