Home /
Expert Answers /
Chemistry /
in-the-following-case-identify-the-more-stable-anion-explain-why-it-is-more-stable-the-anion-pa522
(Solved): In the following case, identify the more stable anion. Explain why it is more stable. The anion ...
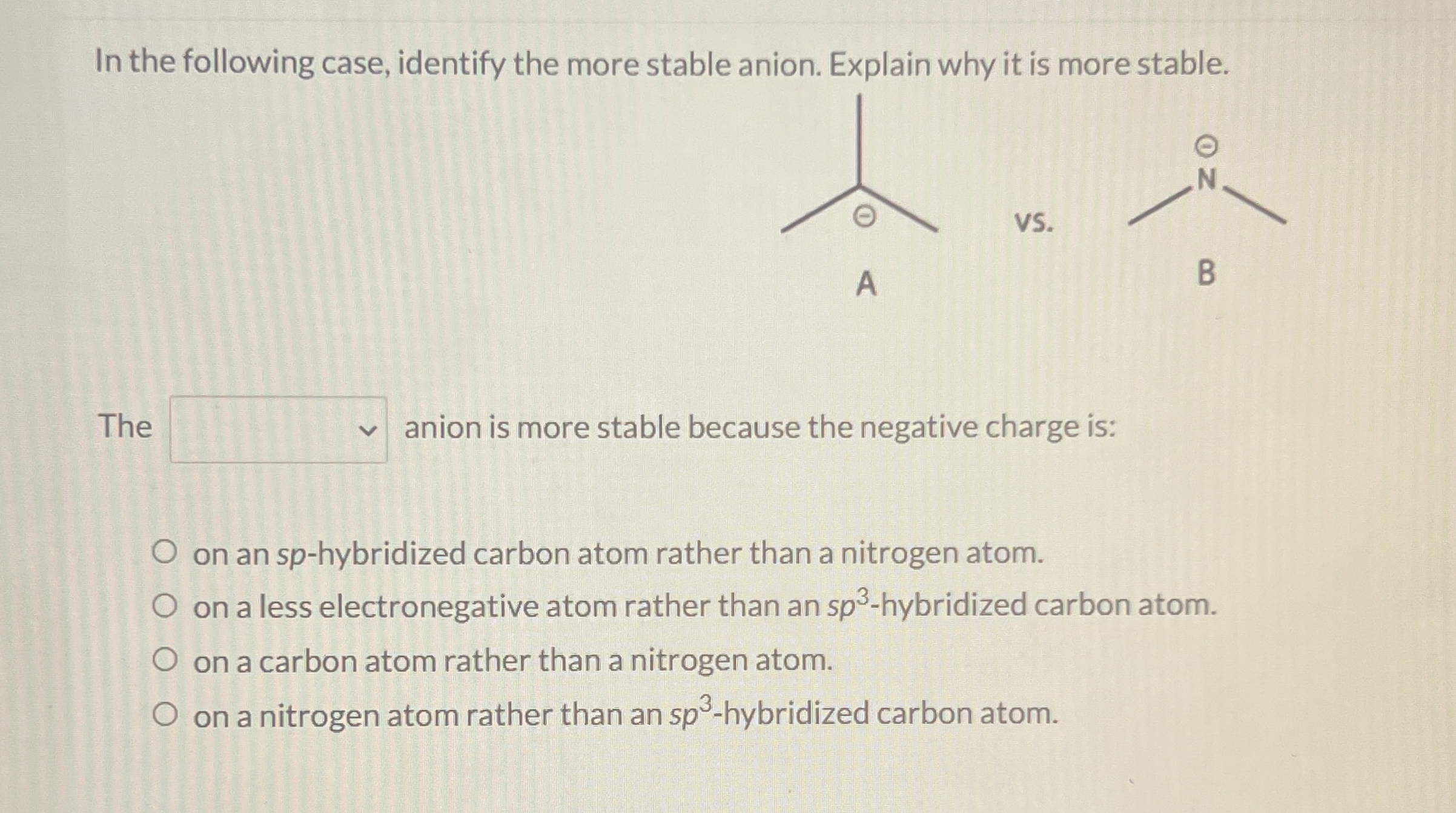
In the following case, identify the more stable anion. Explain why it is more stable. The
?anion is more stable because the negative charge is: on an
sp-hybridized carbon atom rather than a nitrogen atom. on a less electronegative atom rather than an
sp^(3)-hybridized carbon atom. on a carbon atom rather than a nitrogen atom. on a nitrogen atom rather than an
sp^(3)-hybridized carbon atom.