Home /
Expert Answers /
Chemistry /
metallic-aluminum-can-react-with-water-to-create-aluminum-oxide-and-hydrogen-gas-as-shown-in-the-eq-pa723
(Solved): Metallic aluminum can react with water to create aluminum oxide and hydrogen gas as shown in the eq ...
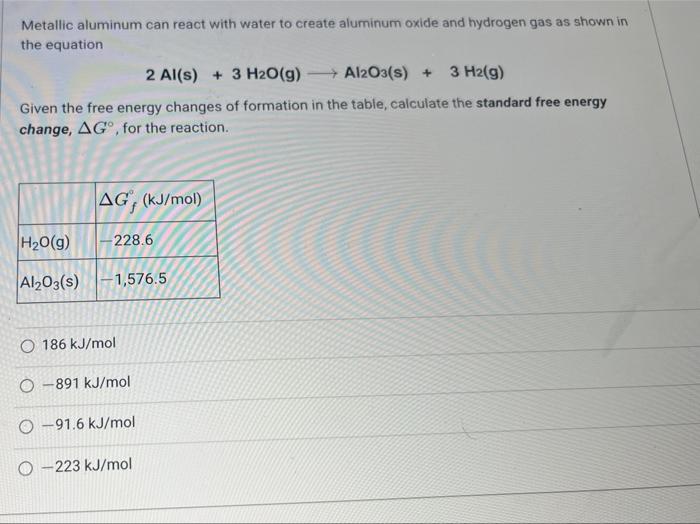
Metallic aluminum can react with water to create aluminum oxide and hydrogen gas as shown in the equation Given the free energy changes of formation in the table, calculate the standard free energy change, , for the reaction.