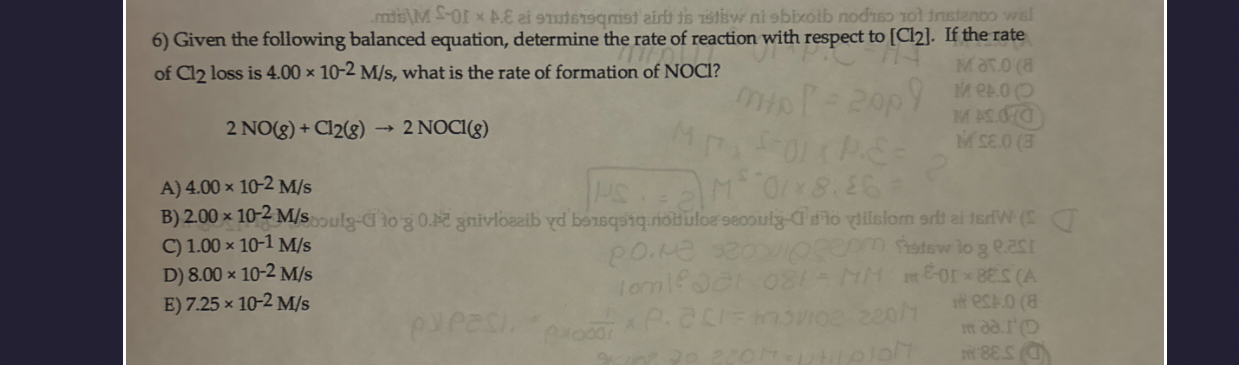(Solved): mistlu S-0I x_(||)_(.)\epsi ai 9 muts Given the following balanced equation, determine the rate of ...
mistlu S-0I
x_(||)
_(.)\epsi ai 9 muts Given the following balanced equation, determine the rate of reaction with respect to
Cl_(2). If the rate of
Cl_(2)loss is
4.00\times 10^(-2)(M)/(s), what is the rate of formation of NOCl ?
2NO(g)+Cl_(2)(g)->2NOCl(g)A)
4.00\times 10^(-2)(M)/(s)B)
2.00\times 10^(-2)(M)/(s)C)
1.00\times 10^(-1)(M)/(s)D)
8.00\times 10^(-2)(M)/(s)E)
7.25\times 10^(-2)(M)/(s)