Home /
Expert Answers /
Chemistry /
nbsp-concentration-as-a-function-of-time-for-the-reaction-of-a-forming-b-check-all-pa116
(Solved): Concentration as a Function of Time for the Reaction of \( A \) forming \( B \) Check all ...
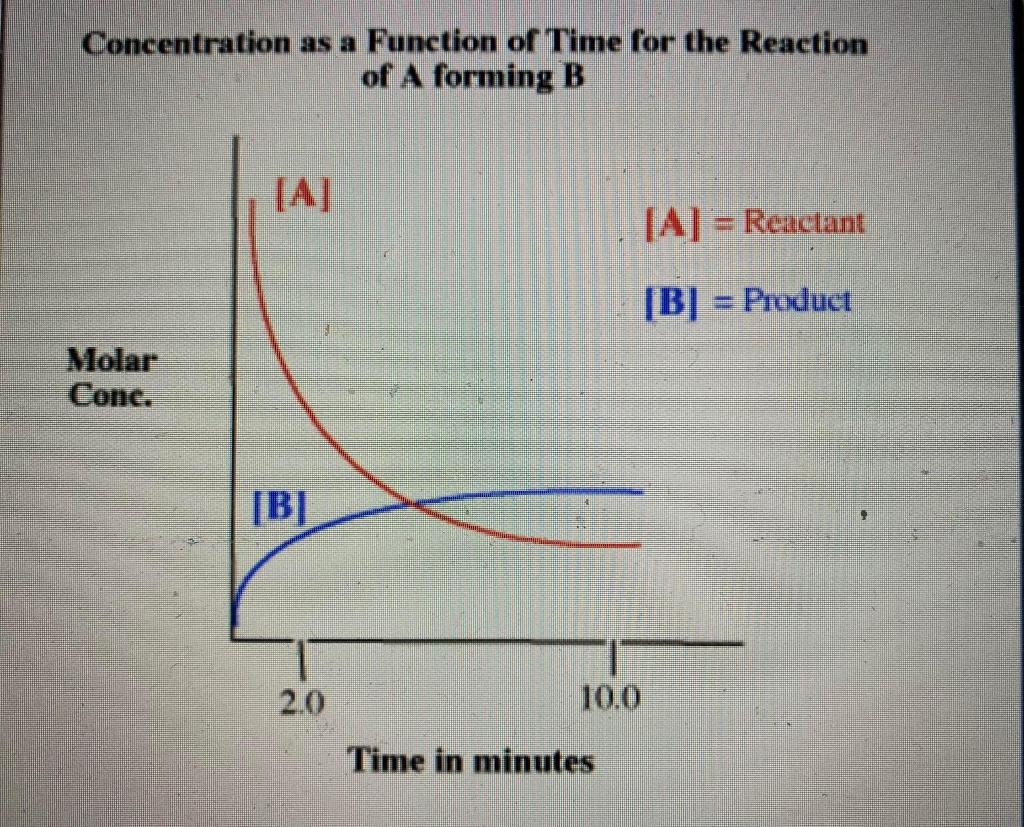
Concentration as a Function of Time for the Reaction of \( A \) forming \( B \)
Check all of the following statements that are true about this diagram and the reaction it represents. Early in the reaction, the rate of the forward reaction is faster than the rate of the reverse reaction. At approximately 5 minutes, the system is at equilibrium because \( [A] \) and \( [B] \) are equal. This graph describes a reaction with a \( K_{e q} \) that is smaller than 1 : At approximately 10 minutes, the system is at equilibrium. At approximately 10 minutes, the rate of formation for the products and reactants are equal. The value of \( \Delta G^{\circ} \) for this reaction is constant. This graph describes a reaction where \( \Delta G^{\circ} \) is greater than zero.
Expert Answer
ANSWERS: 1st statement: Early in the reaction, the rate of the forward reaction is faster than the rate of the reverse reaction. This statement is TRUE, because the slope of the line of concentration of the forward reaction, that represents the rate