Home /
Expert Answers /
Chemistry /
need-help-nbsp-nbsp-not-sure-if-i-did-it-correctly-part-b-determining-the-concentration-pa421
(Solved): need help. not sure if I did it correctly PART B: DETERMINING THE CONCENTRATION ...
need help.

not sure if I did it correctly
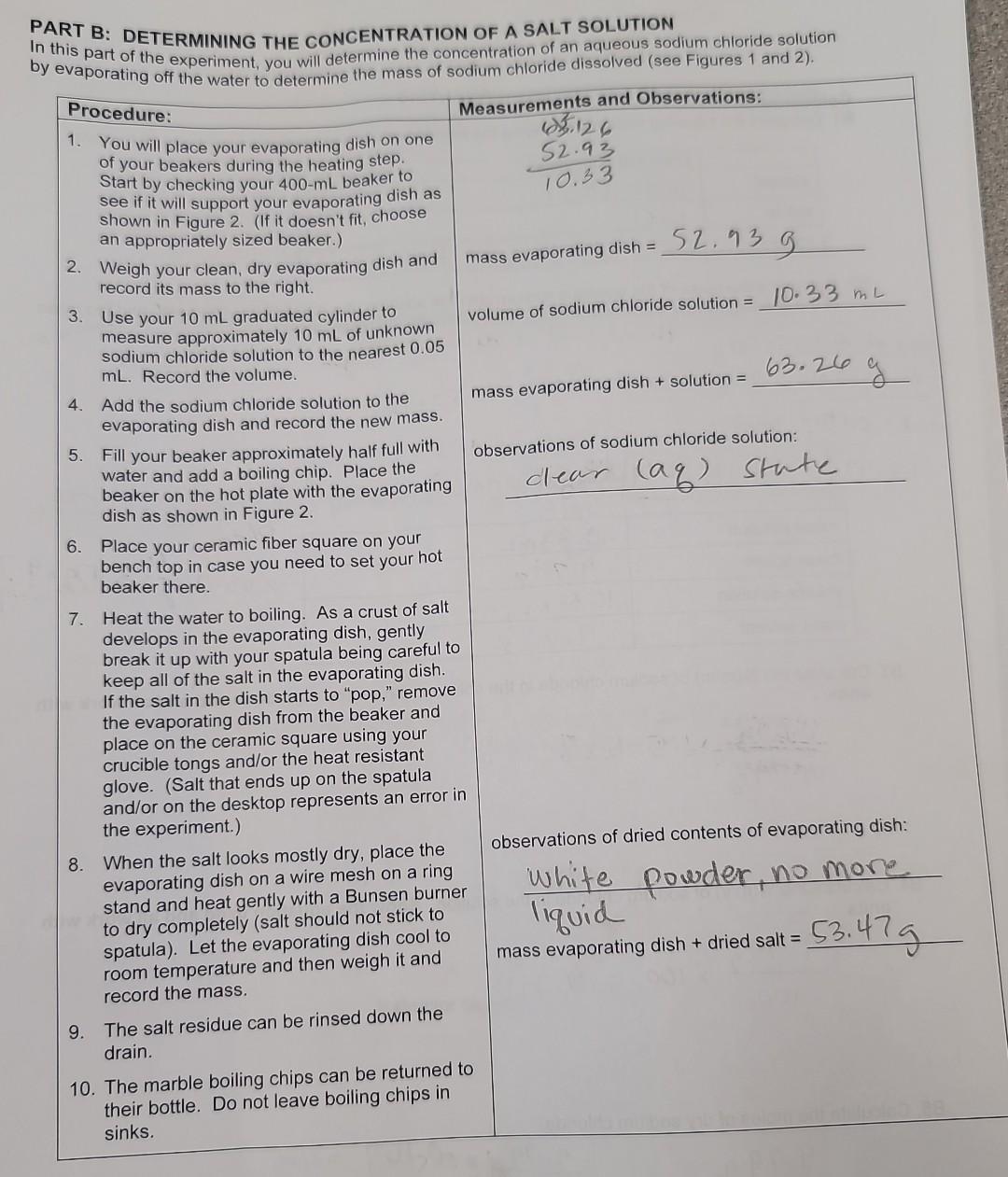
PART B: DETERMINING THE CONCENTRATION OF A SALT SOLUTION In this part of the experiment, you will determine the concentration of an aqueous sodium chloride solution by evaporating off the water, to determine the mass of sodium chloride dissolved (see Figures 1 and 2).
Calculations: B1. Complete the following table by identifying the components of the solution using chemical formulas. B2. Determine the mass of the aqueous sodium chloride solution, the mass of the dried salt, and the mass of the solvent. Show work with units. Then complete the table below. died salt \( + \) dish \( =53.42 g \) Viass of dish:52.93g dish dish \( 10.33 \mathrm{~mL} \) mass of solution \( (a q) \) is \( k+\operatorname{solt} 0 n: 63 \cdot 26 g \) \( 53.47 y-52.93=0.54 \) mass of dried \( \rightarrow 63 \cdot 26 g-53 \cdot 479=9 \) B3. Calculate the \( \%(\mathrm{~m} / \mathrm{m}) \) of sodium chloride in the solution. Show the equation used and all work with units. \[ \frac{0.549}{4.799}=5.5 .2 \% \] B4. Calculate the \( \%(\mathrm{~m} / \mathrm{v}) \) of sodium chloride in the solution. Show the equation used and all work with units. \[ \frac{9.799}{100} \times 100=9.790^{\circ} \] B5. Calculate the moles of dry sodium chloride.
Expert Answer
Solution, Known quantites, mass of evaporating dish = 52.93 g Volume of Sodium Chloride solution = 10 mL. mass of evaporating dish + Solution = 63.26 g. mass of evaporating dish + dried