Home /
Expert Answers /
Chemistry /
need-help-with-all-parts-or-whatever-you-can-use-the-table-to-rationalize-concepts-for-the-followi-pa461
(Solved): Need help with all parts or whatever you can! (Use the table to rationalize concepts)For the followi ...
Need help with all parts or whatever you can! (Use the table to rationalize concepts)
For the following reactions:
- Assign oxidation numbers for each of the elements participating,
- Identify the oxidant and reductant and write the reduction and oxidation half-reactions
- Balance the equations
- Write the net equations in the neutral form (where necessary)
- Determine if the redox processes are spontaneous under standard conditions
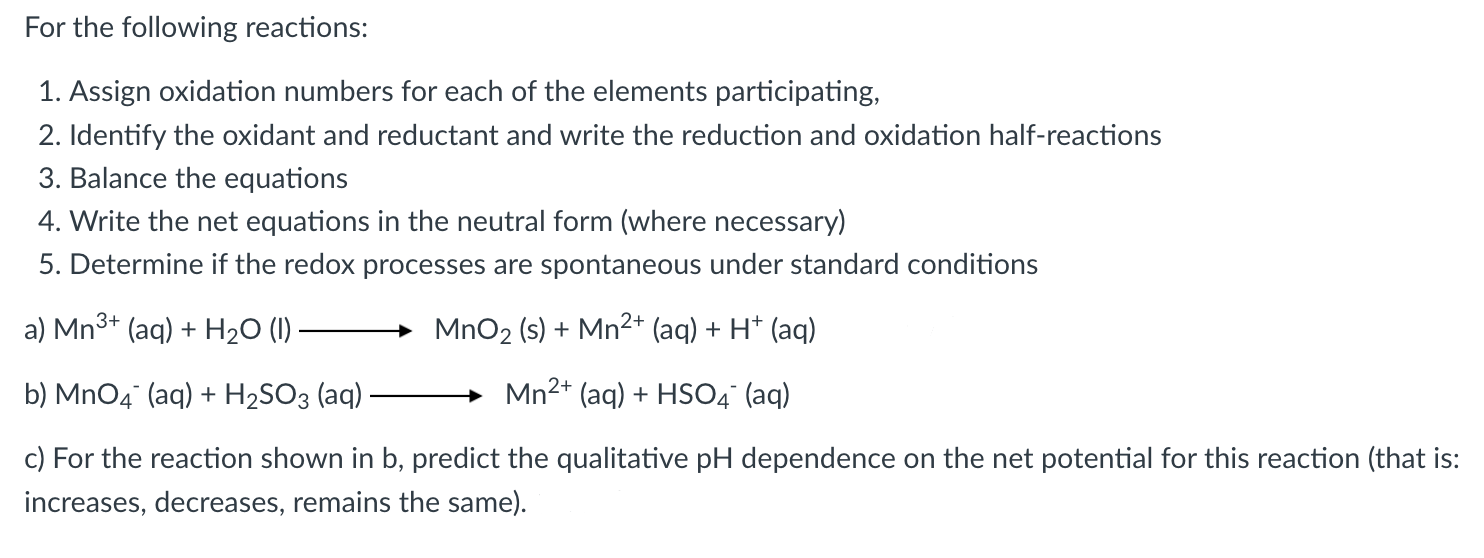
TABLE 18.1 Standard Electrode Potentials at
For the following reactions: 1. Assign oxidation numbers for each of the elements participating, 2. Identify the oxidant and reductant and write the reduction and oxidation half-reactions 3. Balance the equations 4. Write the net equations in the neutral form (where necessary) 5. Determine if the redox processes are spontaneous under standard conditions a) b) (aq) c) For the reaction shown in b, predict the qualitative dependence on the net potential for this reaction (that is: increases, decreases, remains the same).