Home /
Expert Answers /
Chemistry /
nitrogen-dioxide-reacts-with-water-to-form-nitric-acid-and-nitrogen-monoxide-according-to-the-equat-pa860
(Solved): Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equat ...
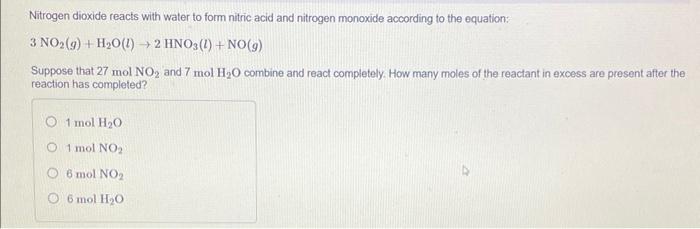
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: \[ 3 \mathrm{NO}_{2}(g)+\mathrm{H}_{2} \mathrm{O}(l) \rightarrow 2 \mathrm{HNO}_{3}(l)+\mathrm{NO}(g) \] Suppose that \( 27 \mathrm{~mol} \mathrm{NO}_{2} \) and \( 7 \mathrm{~mol} \mathrm{H}_{2} \mathrm{O} \) combine and react completely. How many moles of the reactant in excess are present after the reaction has completed? \( 1 \mathrm{~mol} \mathrm{H} \mathrm{H}_{2} \mathrm{O} \) \( 1 \mathrm{~mol} \mathrm{NO}_{2} \) \( 6 \mathrm{~mol} \mathrm{NO}_{2} \) \( 6 \mathrm{~mol} \mathrm{H}_{2} \mathrm{O} \)