Home /
Expert Answers /
Mechanical Engineering /
one-mole-of-an-ideal-monatomic-gas-is-taken-through-the-cycle-shown-to-the-left-assum-that-p-2p0-pa497
(Solved): One mole of an ideal monatomic gas is taken through the cycle shown to the left. Assum that P=2P0 ...
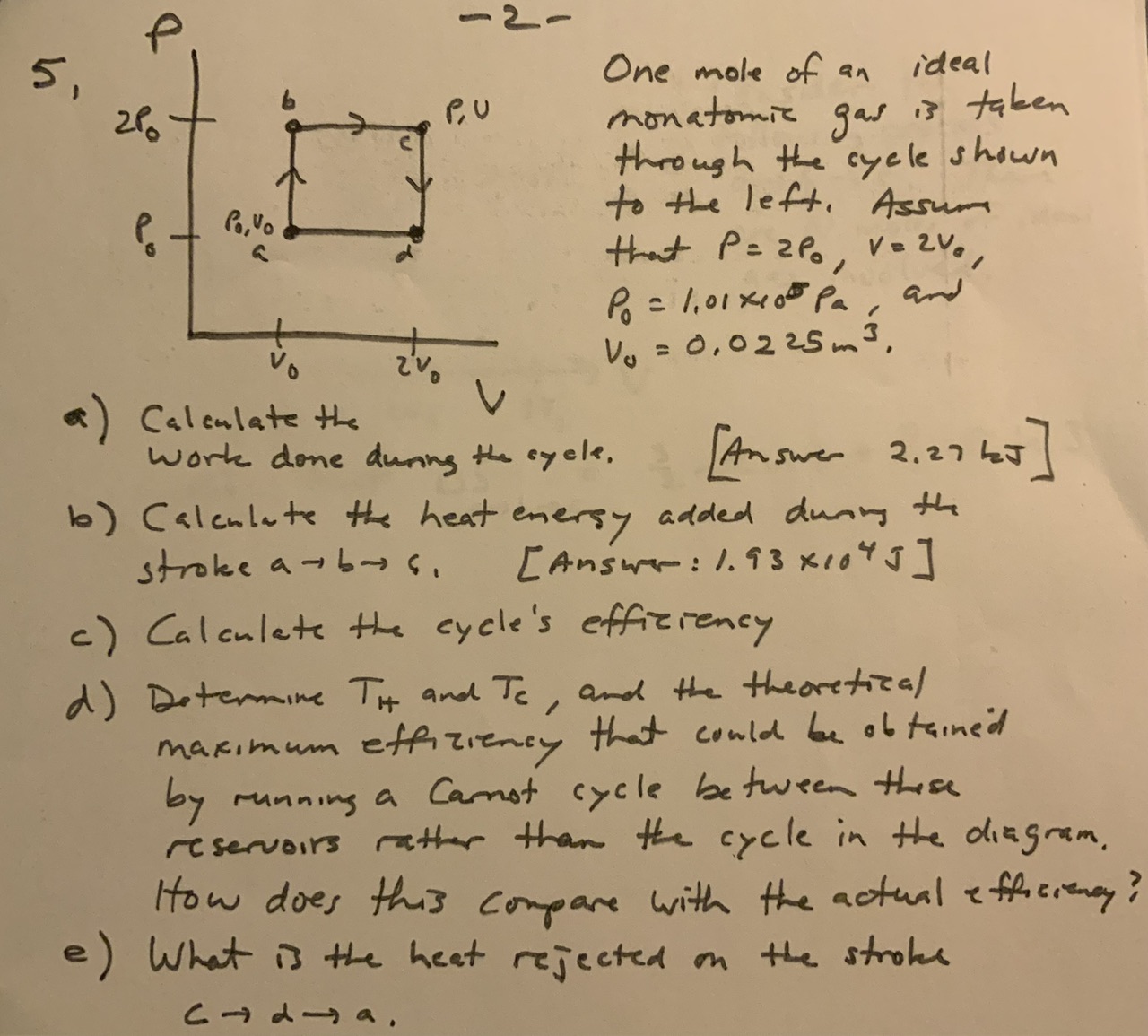
One mole of an ideal monatomic gas is taken through the cycle shown to the left. Assum that , , and . a) Calculate the work done durng the syele. [Answer 2.27 ho] b) Calculate the heat enersy added dunn the stroke . [Answr: ] c) Calculate the eycle's effiziency d) Determine and , and the theoretical maximum effiziency that could be obtained by running a carnot cycle between these reservoirs rather than the cycle in the diagram. How does this conpare with the actual efficingy e) What is the heat rejected on the strole .