Home /
Expert Answers /
Physics /
one-mole-of-ideal-monatomic-gas-e-g-helium-is-taken-along-three-processes-as-shown-in-the-figure-pa921
(Solved): One mole of ideal monatomic gas (e.g. Helium) is taken along three processes as shown in the figure ...
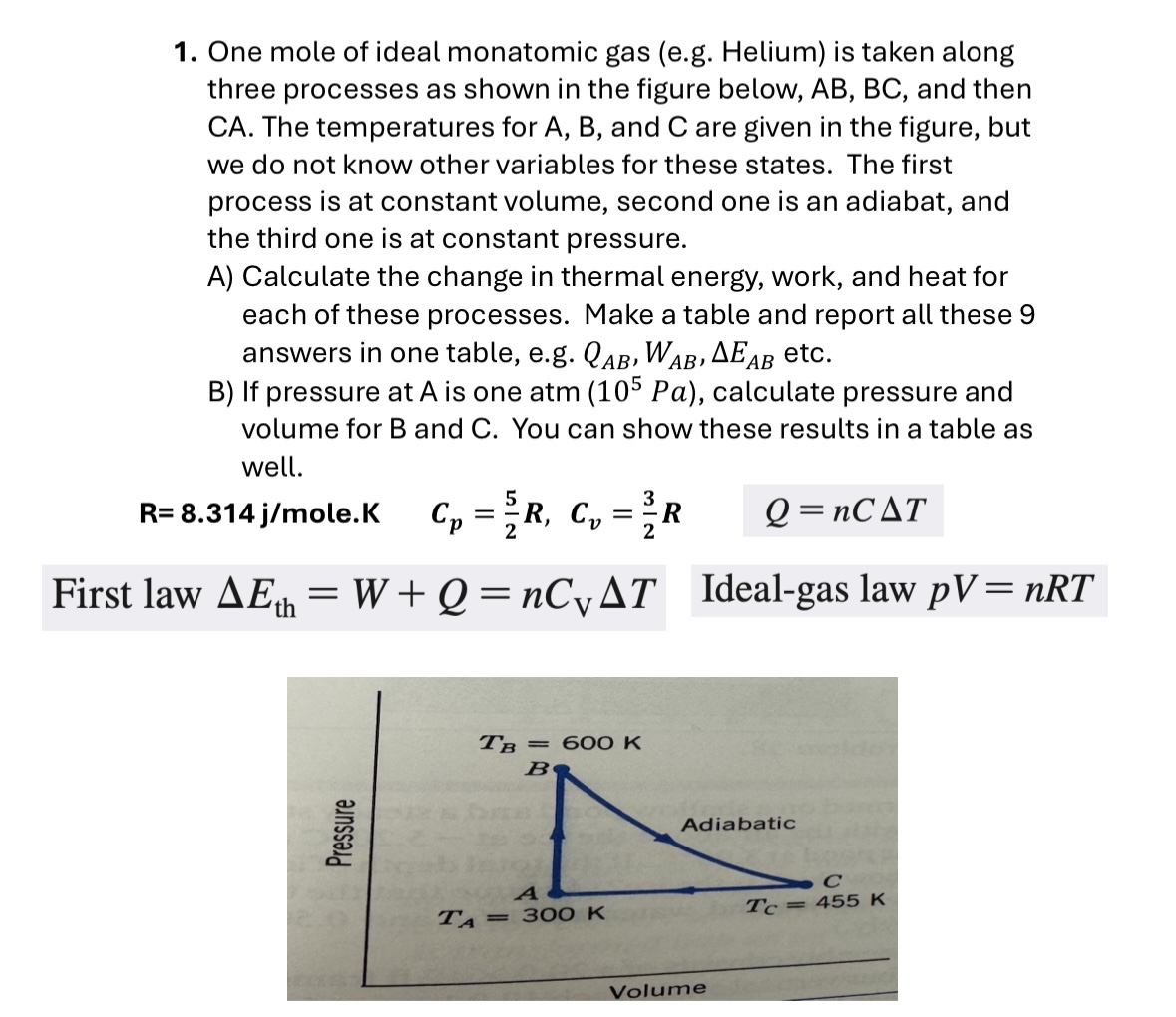
One mole of ideal monatomic gas (e.g. Helium) is taken along three processes as shown in the figure below,
AB,BC, and then
CA. The temperatures for
A,B, and
Care given in the figure, but we do not know other variables for these states. The first process is at constant volume, second one is an adiabat, and the third one is at constant pressure. A) Calculate the change in thermal energy, work, and heat for each of these processes. Make a table and report all these 9 answers in one table, e.g.
Q_(AB),W_(AB),\Delta E_(AB)etc. B) If pressure at A is one
atm(10^(5)(Pa)), calculate pressure and volume for B and C . You can show these results in a table as well.
R=8.314(j)/(m)ole.K,C_(p)=(5)/(2)R,C_(v)=(3)/(2)R,Q=nC\Delta TFirst law
\Delta E_(th)=W+Q=nC_(V)\Delta T,Ideal-gas law
pV=nRT