Home /
Expert Answers /
Chemistry /
part-a-ionic-radii-in-an-isoelectronic-series-put-the-following-atoms-and-ions-in-order-of-increas-pa484
(Solved): Part A - Ionic Radii in an Isoelectronic Series Put the following atoms and ions in order of increas ...
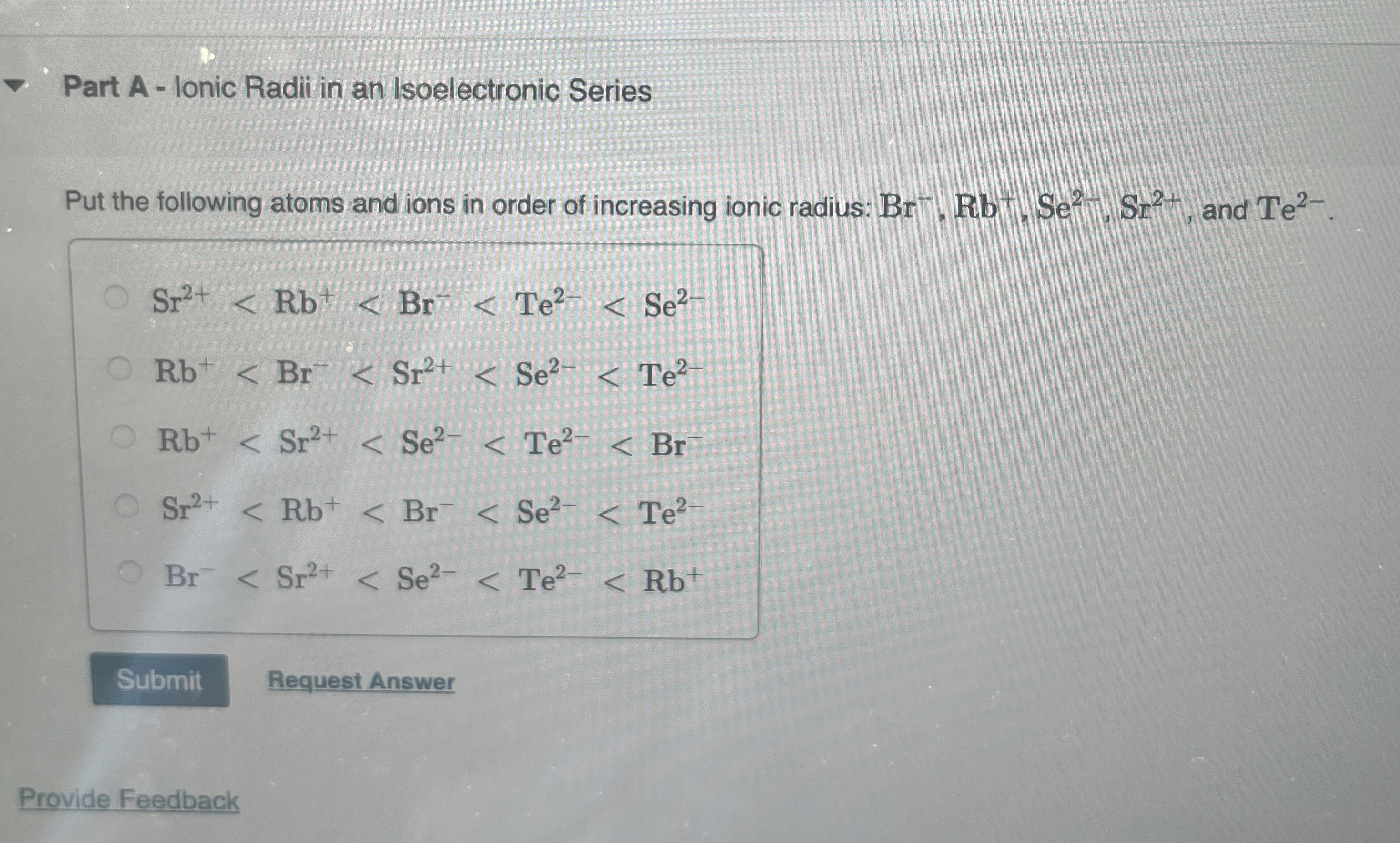
Part A - Ionic Radii in an Isoelectronic Series Put the following atoms and ions in order of increasing ionic radius:
Br^(-),Rb^(+),Se^(2-),Sr^(2+), and
Te^(2-).
Sr^(2+)
Provide Feedback