Home /
Expert Answers /
Chemistry /
please-answer-all-parts-a-and-ba-b-which-of-the-following-aqueous-solutions-are-good-buffer-systems-pa695
(Solved): Please answer all parts A and BA)B) Which of the following aqueous solutions are good buffer systems ...
Please answer all parts A and B

A)
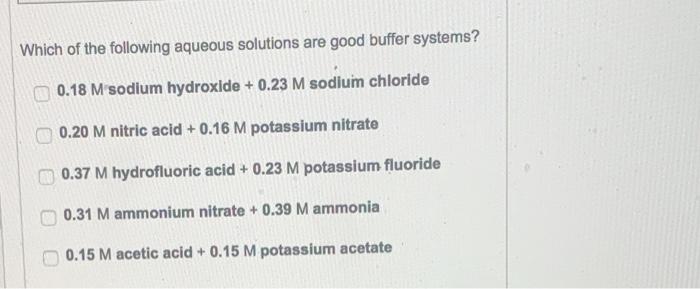

B)

Which of the following aqueous solutions are good buffer systems? \( 0.18 \mathrm{M} \) sodium hydroxide \( +0.23 \mathrm{M} \) sodium chloride \( 0.20 \mathrm{M} \) nitric acid \( +0.16 \mathrm{M} \) potassium nitrate \( 0.37 \mathrm{M} \) hydrofluoric acid \( +0.23 \mathrm{M} \) potassium fluoride \( 0.31 \mathrm{M} \) ammonium nitrate \( +0.39 \mathrm{M} \) ammonia \( 0.15 \mathrm{M} \) acetic acid \( +0.15 \mathrm{M} \) potassium acetate
Which of the following conditions is/are met at the equivalence point of the titration of a monoprotic weak base with a strong acid? 1. The moles of acid added from the buret equals the initial moles of weak base. 2. The volume of acid added from the buret must equal the volume of base titrated. 3. The \( \mathrm{pH} \) of the solution is less than \( 7.00 \). 2 and 3 1 and 3 3 only 1 only 2 only