Home /
Expert Answers /
Chemistry /
please-do-both-parts-nbsp-solid-iron-ii-carbonate-and-solid-cobalt-ii-carbonate-are-in-equilibriu-pa147
(Solved): please do both parts Solid iron(II) carbonate and solid cobalt(II) carbonate are in equilibriu ...
please do both parts



Solid iron(II) carbonate and solid cobalt(II) carbonate are in equilibrium with a solution containing \( 1.06 \times 10^{-2} \mathrm{M} \) iron(II) acetate. Calculate the concentration of cobalt(II) ion present in this solution. \( [ \) cobalt \( (\mathrm{II})]= \)
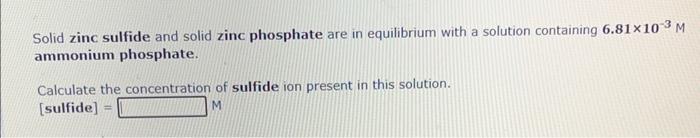
Solid zinc sulfide and solid zinc phosphate are in equilibrium with a solution containing \( 6.81 \times 10^{-3} \mathrm{M} \) ammonium phosphate. Calculate the concentration of sulfide ion present in this solution. [sulfide] \( = \)