Home /
Expert Answers /
Chemistry /
please-help-nbsp-how-many-molecules-does-85-0-mathrm-g-mathrm-hcl-contain-2-04-pa636
(Solved): please help!!!! How many molecules does \( 85.0 \mathrm{~g} \mathrm{HCl} \) contain? \( 2.04 \ ...
please help!!!!
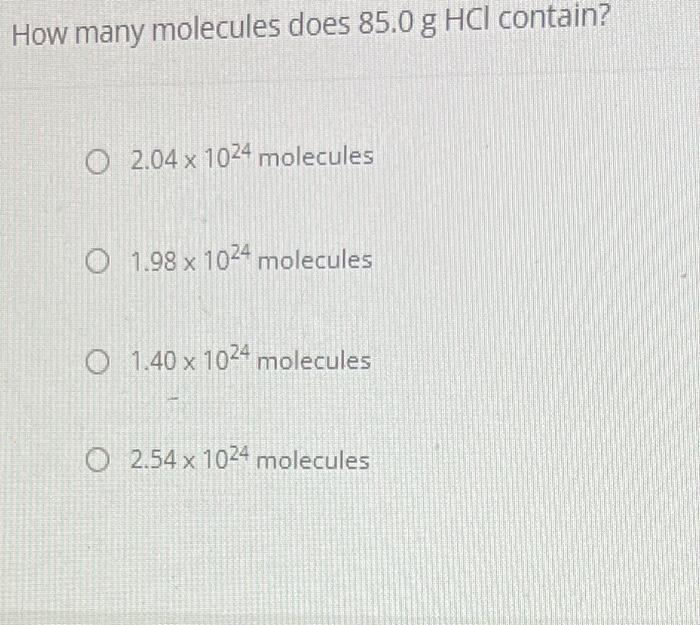

How many molecules does \( 85.0 \mathrm{~g} \mathrm{HCl} \) contain? \( 2.04 \times 10^{24} \) molecules \( 1.98 \times 10^{24} \) molecules \( 1.40 \times 10^{24} \) molecules \( 2.54 \times 10^{24} \) molecules
Expert Answer
Ans- option c- 1.40 x 1024 molecules Given- molar mass of HCl = 36.458 g/mol mass of HCl= 85