Home /
Expert Answers /
Chemistry /
pls-answer-fill-out-the-table-and-asnwer-the-questions-based-off-of-the-data-provided-in-red-on-pa588
(Solved): Pls answer!! fill out the table and asnwer the questions based off of the data provided in red. ( on ...
Pls answer!! fill out the table and asnwer the questions based off of the data provided in red. ( on paper is best)
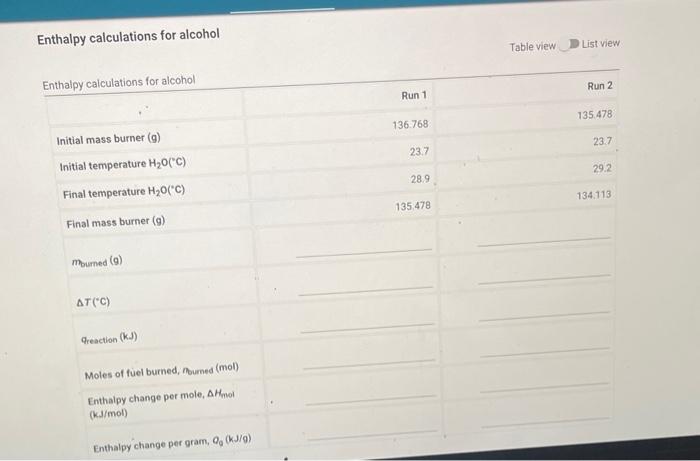





Enthalpy calculations for alcohol Table view \( \boldsymbol{D} \) List view
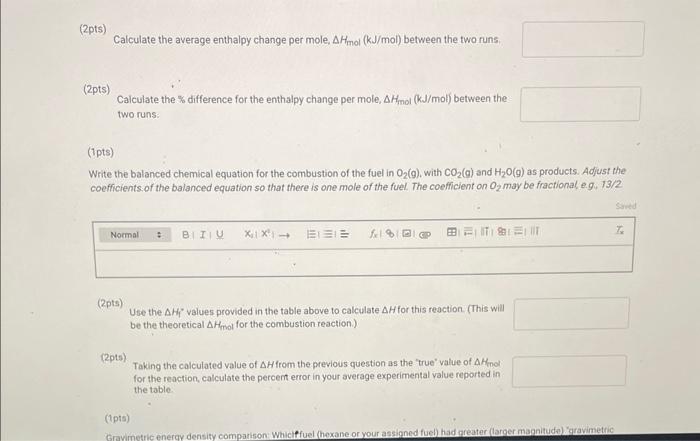
(2pts) Calculate the average enthalpy change per mole, \( \Delta H_{\mathrm{mol}}(\mathrm{kJ} / \mathrm{mol}) \) between the two runs. (2pts) Calculate the \% difference for the enthalpy change per mole, \( \Delta H_{\text {mol }}(\mathrm{kJ} / \mathrm{mol}) \) between the two runs: (7pts) Write the balanced chemical equation for the combustion of the fuel in \( \mathrm{O}_{2}(g) \), with \( \mathrm{CO}_{2}(\mathrm{~g}) \) and \( \mathrm{H}_{2} \mathrm{O}(g) \) as products. Adjust the coefficients. of the balanced equation so that there is one mole of the fuel. The coefficient on \( \mathrm{O}_{2} \) may be fractional eg. \( 13 / 2 \) \( (2 p t s) \) Use the \( \Delta H_{f}^{*} \) values provided in the table above to calculate \( \Delta H \) for this reaction. (This will be the theoretical \( \Delta H_{\text {mol }} \) for the combustion reaction.) (2pt5) Taking the calculated value of \( \Delta H \) from the previous question as the "true' value of \( \Delta H \) fol for the reaction, calculate the percent error in your average experimental value reported in . the table.
Gravimetric energy density comparison: Which fuel (hexane or your assigned fuel) had greater (larger magnitude) "gravimetric energy density. i.e. provided the most energy per gram (same as \( Q_{g} \) )? Does this make sense? Explain briefly. \( ( \) Opts) Upload an image showing your work for the calculations. Make sure the calculations are labeled appropriately.
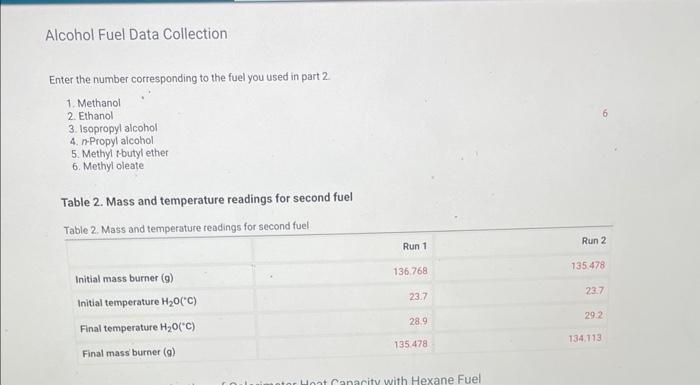
Enter the number corresponding to the fuel you used in part 2. 1. Methanol 2. Ethanol 3. Isopropyl alcohol 4. n-Propyl alcohol 5. Methyl t-butyl ether 6. Methyl oleate Table 2. Mass and temperature readings for second fuel
Expert Answer
Run1. mass burned= 136.768-135.478 = 1.29 g delta T = 28.9-23.7 = 5.2 C = 278.2K At ambient pressure and temperature, the isobaric specific heat, CP,