Home /
Expert Answers /
Chemistry /
plz-help-for-a-thumbs-up-and-good-review-according-to-the-following-reaction-how-many-grams-of-iron-pa236
(Solved): PLZ HELP FOR A THUMBS UP AND GOOD REVIEW According to the following reaction, how many grams of iron ...
PLZ HELP FOR A THUMBS UP AND GOOD REVIEW
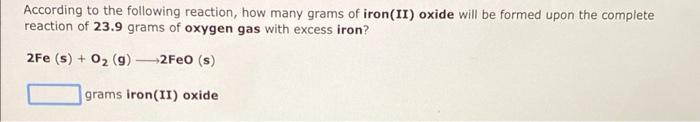


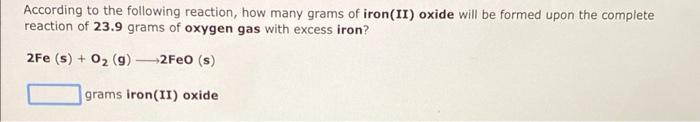


According to the following reaction, how many grams of iron(II) oxide will be formed upon the complete reaction of \( 23.9 \) grams of oxygen gas with excess iron? \[ 2 \mathrm{Fe}(\mathrm{s})+\mathrm{O}_{2}(\mathbf{g}) \longrightarrow 2 \mathrm{FeO}(\mathrm{s}) \] grams iron(II) oxide
According to the following reaction, how many grams of dinitrogen monoxide will be formed upon the complete reaction of \( \mathbf{2 1 . 7} \) grams of ammonium nitrate? \[ \mathrm{NH}_{4} \mathrm{NO}_{3}(\mathrm{aq}) \longrightarrow \mathrm{N}_{2} \mathrm{O}(g)+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \] grams dinitrogen monoxide
For the following reaction, \( 3.69 \) grams of phosphoric acid are mixed with excess potassium hydroxide. The reaction yields \( 6.92 \) grams of potassium phosphate. \[ 3 \mathrm{KOH}(\mathrm{aq})+\mathrm{H}_{3} \mathrm{PO}_{4}(\mathrm{aq}) \longrightarrow \mathrm{K}_{3} \mathrm{PO}_{4}(\mathrm{aq})+3 \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \] (1) What is the theoretical yield of potassium phosphate? grams (2) What is the percent yield for this reaction?
Expert Answer
Solution:- Molar mass of oxygen = 32 amu Molar mass of FeO = 71.85 amu 2Fe(s) + O2(g) ------>2FeO(s) Number of moles of oxygen = given mass / molar mass =23.9/32 =0.7465g So, 0.7465 moles of oxygen requir