Home /
Expert Answers /
Chemistry /
protons-neutrons-electrons-ions-isotopes-name-directions-fill-in-the-table-below-table-tab-pa416
(Solved): Protons, Neutrons, Electrons, Ions, Isotopes Name: Directions: Fill in the table below. \table[[\tab ...
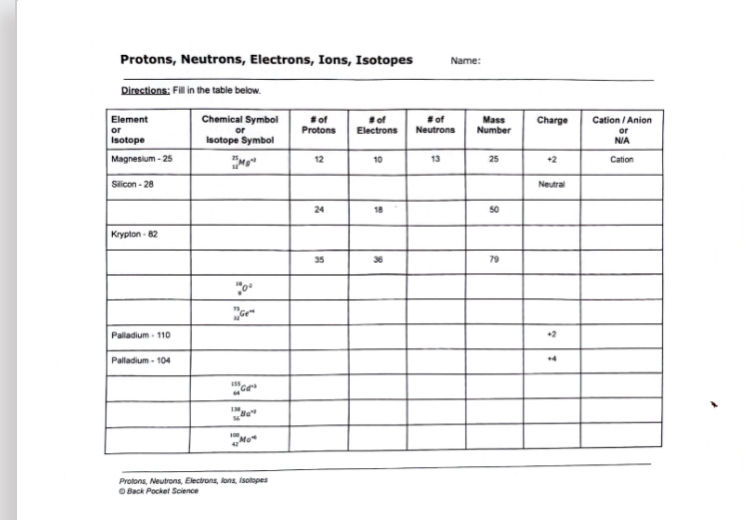
Protons, Neutrons, Electrons, Ions, Isotopes Name: Directions: Fill in the table below. \table[[\table[[Element],[or],[Isotope]],\table[[Chemical Symbol],[or],[Isotope Symbol]],\table[[E of],[Protons]],\table[[3 of],[Electrons]],\table[[E of],[Neutrons]],\table[[Mass],[Number]],Charge,\table[[Cation / Anion],[or],[N/A]]],[Magnesium - 25,
_(4)^(_(4)^(3))M_(g)g^(-4),12,10,13,25,+2,Cation],[Silicon - 28,,,,,,Neutral,],[,24,18,,50,,],[Krypton - 82],[,,35,36,,79,,],[,
^(48)0^(2),,,,,,],[,
_(4)^(n)Ge^(-4),,,,,,],[Palladium - 110,,,,,,+2,],[Palladium -104,,,,,,44,],[
_(4)^(45)Gd^(-4),,,,,,],[
_(5)^(138)8a^(-4),,,,,,],[
_(48)^(100)Mo^(-4),,,,,,]] Protons, Neutrons, Electrons, ians, isoltges O Back Pocket Science