Home /
Expert Answers /
Chemistry /
q20-a-schematic-presentation-of-the-suppression-process-used-in-an-anion-exchange-chromatography-w-pa280
(Solved): Q20. A schematic presentation of the suppression process used in an anion-exchange chromatography w ...
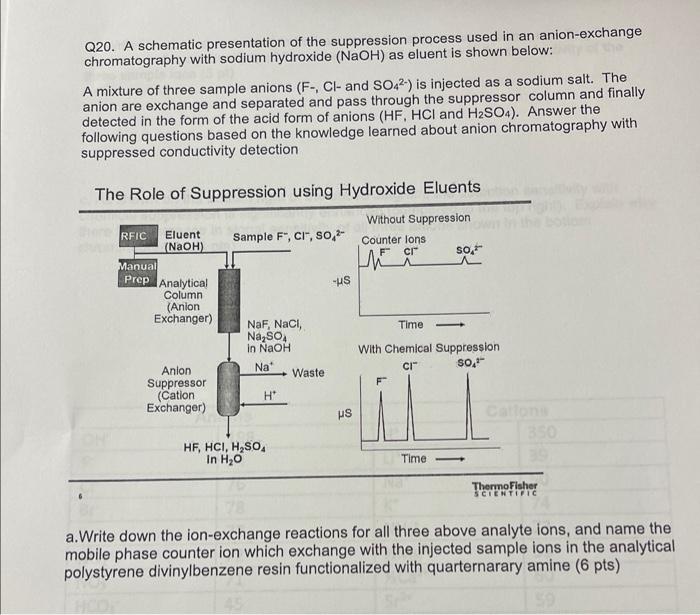
Q20. A schematic presentation of the suppression process used in an anion-exchange chromatography with sodium hydroxide \( (\mathrm{NaOH}) \) as eluent is shown below: A mixture of three sample anions \( \left(\mathrm{F}-, \mathrm{Cl}-\right. \) and \( \left.\mathrm{SO}_{4}^{2-}\right) \) is injected as a sodium salt. The anion are exchange and separated and pass through the suppressor column and finally detected in the form of the acid form of anions \( \left(\mathrm{HF}, \mathrm{HCl}\right. \) and \( \left.\mathrm{H}_{2} \mathrm{SO}_{4}\right) \). Answer the following questions based on the knowledge learned about anion chromatography with suppressed conductivity detection The Role of Suppression using Hydroxide Eluents a.Write down the ion-exchange reactions for all three above analyte ions, and name the mobile phase counter ion which exchange with the injected sample ions in the analytical polystyrene divinylbenzene resin functionalized with quarternarary amine (6 pts)
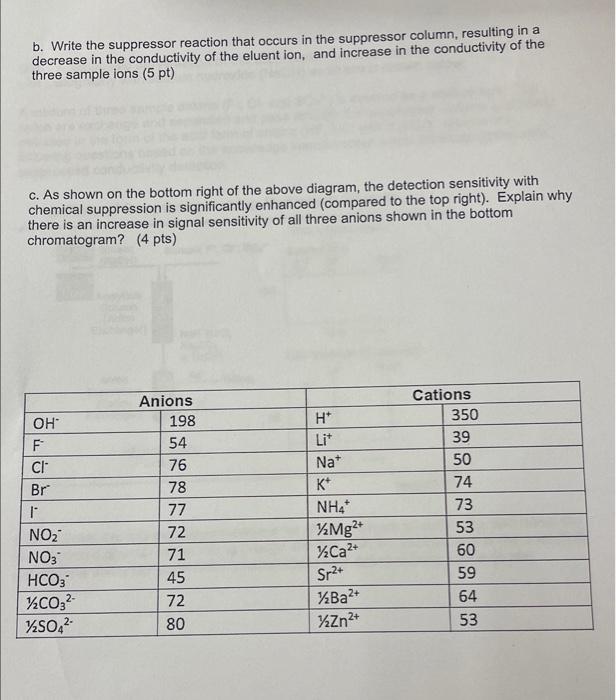
b. Write the suppressor reaction that occurs in the suppressor column, resulting in a decrease in the conductivity of the eluent ion, and increase in the conductivity of the three sample ions (5 pt) c. As shown on the bottom right of the above diagram, the detection sensitivity with chemical suppression is significantly enhanced (compared to the top right). Explain why there is an increase in signal sensitivity of all three anions shown in the bottom chromatogram? (4 pts)
Expert Answer
Dear student, I have provided you