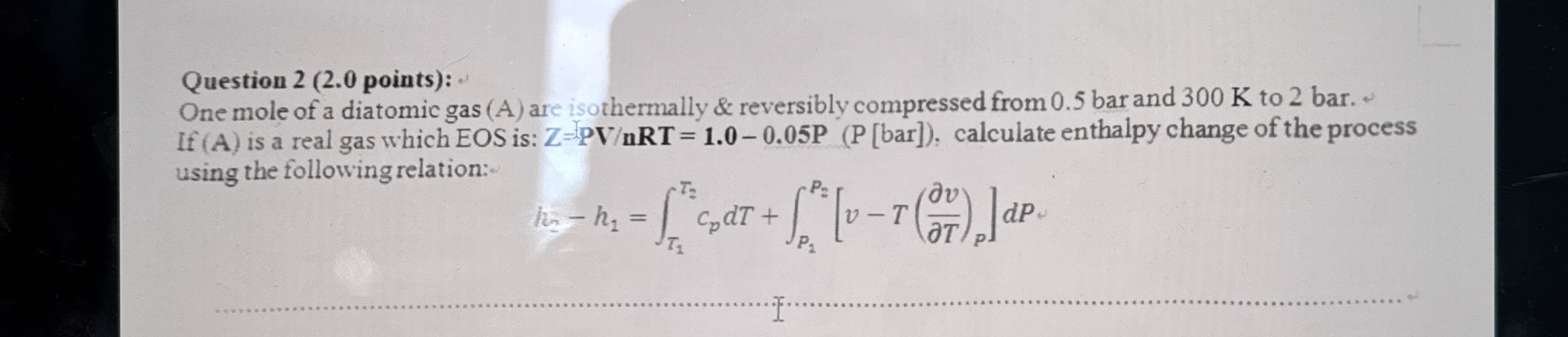Home /
Expert Answers /
Chemical Engineering /
question-2-2-0-points-one-mole-of-a-diatomic-gas-a-are-isothermally-amp-reversibly-compressed-pa971
(Solved): Question 2 (2.0 points): One mole of a diatomic gas (A) are isothermally & reversibly compressed ...
Question 2 (2.0 points): One mole of a diatomic gas (A) are isothermally & reversibly compressed from 0.5 bar and 300 K to 2 bar. If
(A)is a real gas which
EOSis:
Z=P(V)/(R)T=1.0-0.05P(P[/bar ]), calculate enthalpy change of the process using the following relation:
h_(2)-h_(1)=\int_(T_(1))^(T_(2)) c_(p)dT+\int_(P_(2))^(P_(2)) [v-T((delv)/(delT))_(P)]dP