Home /
Expert Answers /
Chemistry /
question-8-1-pts-give-the-molecular-equation-for-the-reaction-if-any-that-occurs-when-aqueous-solu-pa328
(Solved): Question 8 1 pts Give the molecular equation for the reaction (if any) that occurs when aqueous solu ...
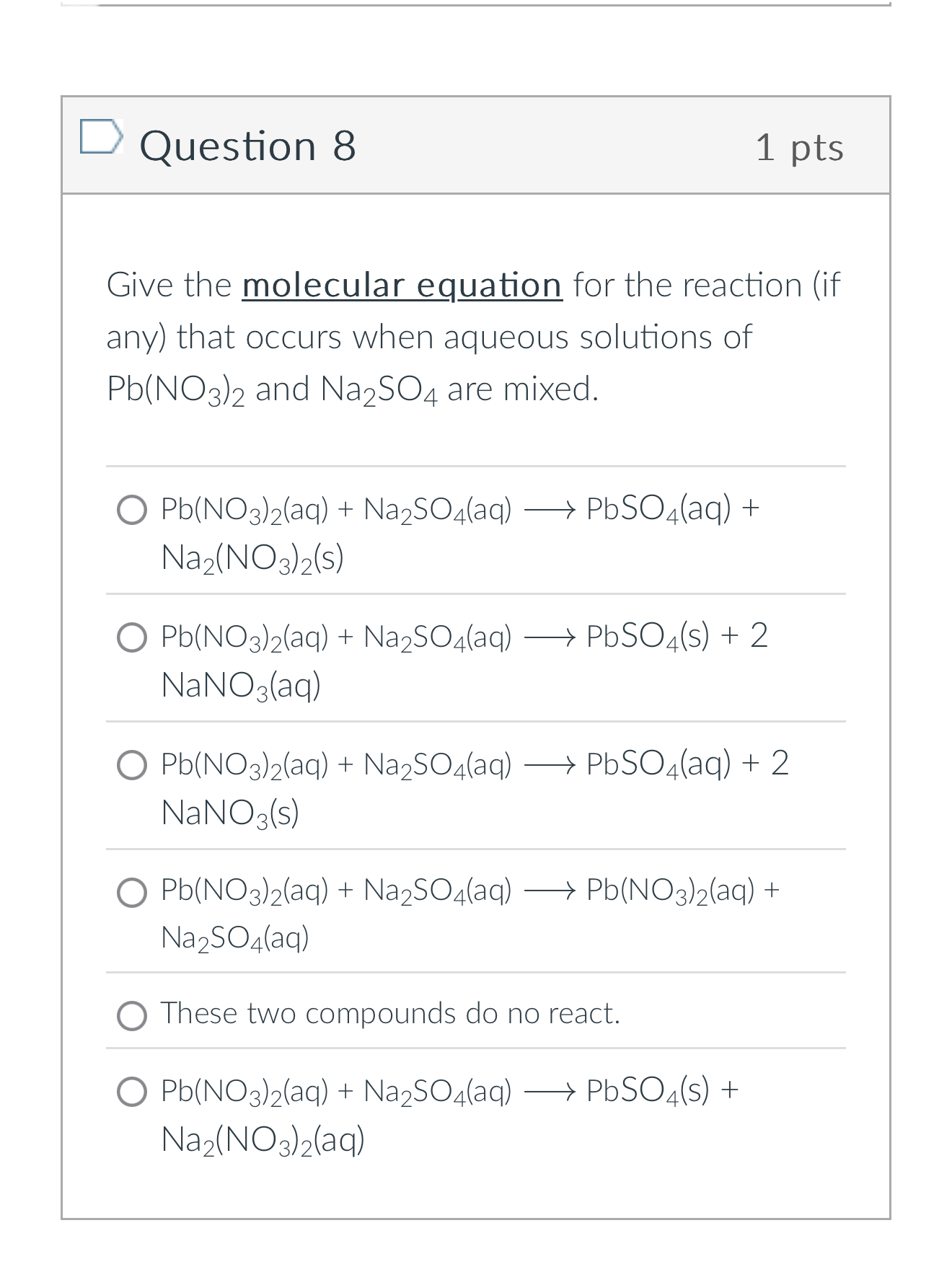
Question 8 1 pts Give the molecular equation for the reaction (if any) that occurs when aqueous solutions of
Pb(NO_(3))_(2)and
Na_(2)SO_(4)are mixed.
Pb(NO_(3))_(2)(aq)+Na_(2)SO_(4)(aq)longrightarrowPbSO_(4)(aq)+
Na_(2)(NO_(3))_(2)(s)
Pb(NO_(3))_(2)(aq)+Na_(2)SO_(4)(aq)longrightarrowPbSO_(4)(s)+2
NaNO_(3)(aq)
Pb(NO_(3))_(2)(aq)+Na_(2)SO_(4)(aq)longrightarrowPbSO_(4)(aq)+2
NaNO_(3)(s)
Pb(NO_(3))_(2)(aq)+Na_(2)SO_(4)(aq)longrightarrowPb(NO_(3))_(2)(aq)+
Na_(2)SO_(4)(aq)These two compounds do no react.
Pb(NO_(3))_(2)(aq)+Na_(2)SO_(4)(aq)longrightarrowPbSO_(4)(s)+
Na_(2)(NO_(3))_(2)(aq)