Home /
Expert Answers /
Chemistry /
representing-the-acid-as-ha-nbsp-analysis-1-representing-the-acid-as-ha-a-monoprotic-acid-so-it-r-pa711
(Solved): representing the acid as HA Analysis 1. Representing the acid as HA (a monoprotic acid so it r ...
representing the acid as HA
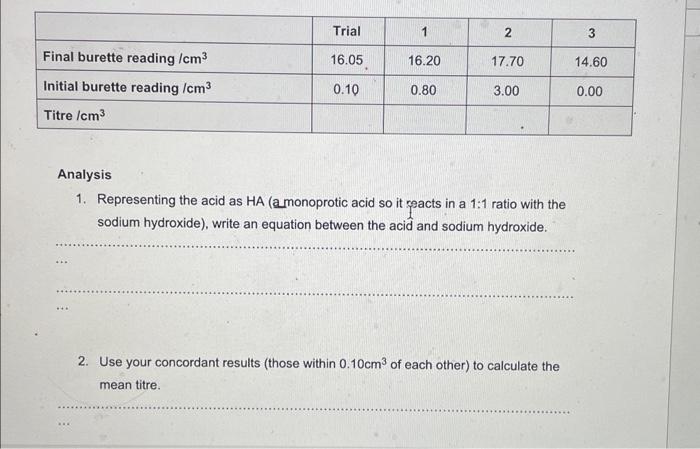



Analysis 1. Representing the acid as HA (a monoprotic acid so it reacts in a \( 1: 1 \) ratio with the sodium hydroxide), write an equation between the acid and sodium hydroxide. 2. Use your concordant results (those within \( 0.10 \mathrm{~cm}^{3} \) of each other) to calculate the mean titre.
3. Calculate the amount in moles of \( \mathrm{NaOH} \) in the \( 25.0 \mathrm{~cm}^{3} \) used in each titration. 4. Using the equation above, write down the amount in moles of HA in your mean titre. 5. Calculate the amount in moles of \( \mathrm{HA} \) in \( 250 \mathrm{~cm}^{3} \) of the acid solution
Expert Answer
1. The reaction between the acid and sod