Home /
Expert Answers /
Chemical Engineering /
sample-problem-surface-tension-of-water-at-20c-is-7-27102nm1-and-its-density-is-0-998-pa747
(Solved): Sample problem: Surface tension of water at 20C is 7.27102Nm1 and its density is 0.998 ...
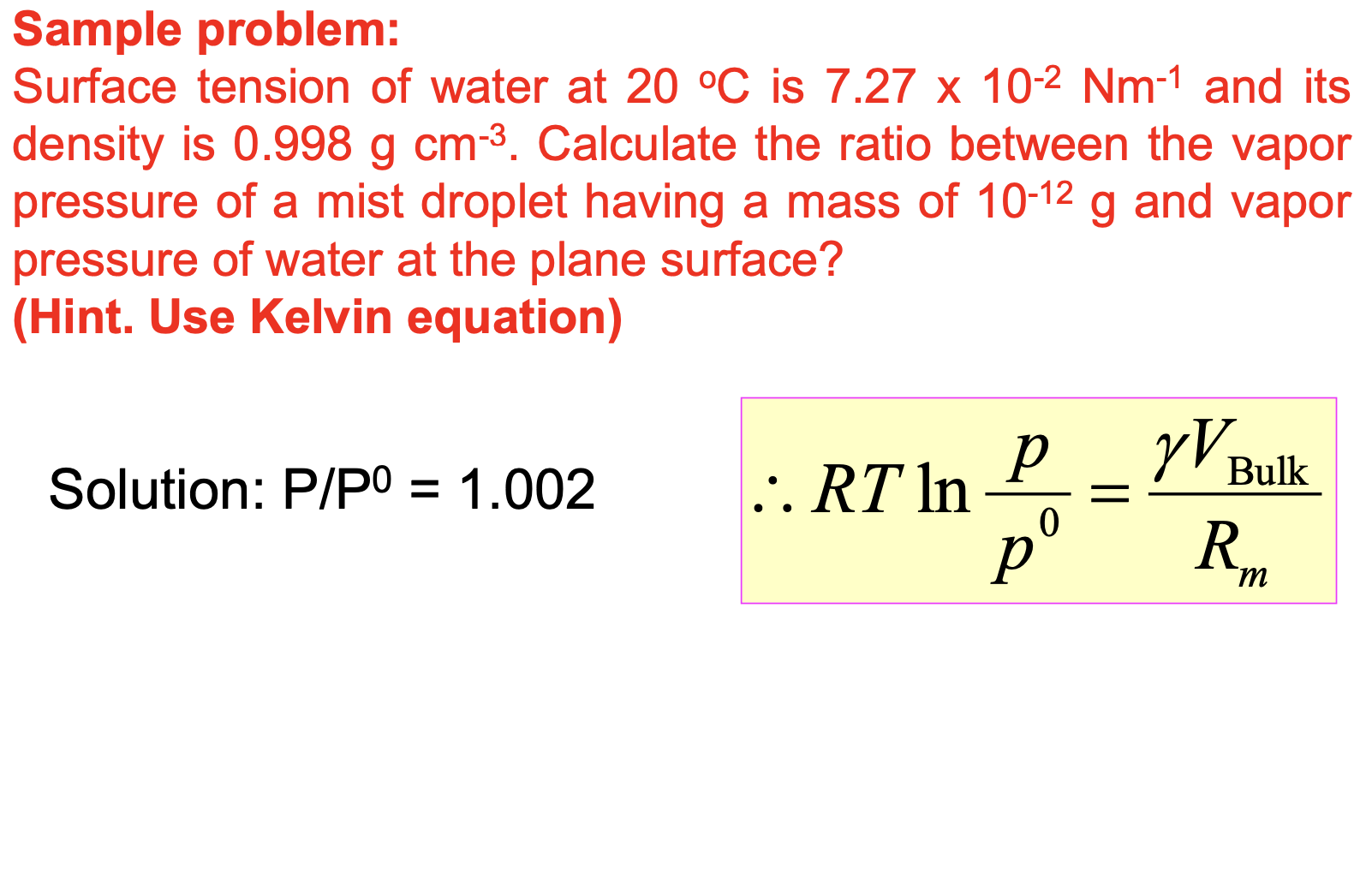
Sample problem: Surface tension of water at is and its density is . Calculate the ratio between the vapor pressure of a mist droplet having a mass of and vapor pressure of water at the plane surface? (Hint. Use Kelvin equation) Solution:
Expert Answer
To calculate the ratio between the vapor pressure of a mist droplet and the vapor pressure of water at the plane surface, we can use the Kelvin equation, which relates the vapor pressure of a droplet to the vapor pressure of the plane surface based on the surface tension and density of the liquid. The Kelvin equation is given by: where: P is the vapor pressure of the droplet P_0 is the vapor pressure of the plane surface M is the molar mass of the liquid (in kg/mol) is the surface tension of the liquid (in N/m) ? is the density of the liquid (in kg/m^3) R is the gas constant (8.314 J/(mol·K)) T is the temperature in Kelvin r is the radius of the droplet