Home /
Expert Answers /
Chemistry /
table-formula-is-compound-and-acid-base-or-salt-is-an-aqueous-solution-of-the-compound-acidic-pa434
(Solved): \table[[Formula,Is Compound and acid, base, or salt?,Is an aqueous solution of the compound acidic, ...
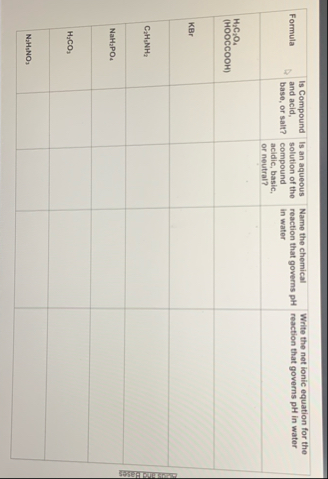
\table[[Formula,Is Compound and acid, base, or salt?,Is an aqueous solution of the compound acidic, basic, or neutral?,Name the chemical reaction that governs pH in water,Write the net ionic equation for the reaction that governs pH in water],[
H_(2)C_(2)O_(4)( HOOCCOOH ),,,,],[KBr,,,,],[
C_(2)H_(5)NH_(2),,,,],[
NaH_(2)PO_(4),,,,],[
H_(3)CO_(3),,,,],[
N_(2)H_(3)NO_(3),,,,]]