Home /
Expert Answers /
Physics /
the-ammonia-molecule-nh-3-has-a-dipole-moment-of-5-0-times-10-30-c-m-ammonia-molecules-in-the-pa715
(Solved): The ammonia molecule (NH_(3)) has a dipole moment of 5.0\times 10^(-30)C*m Ammonia molecules in the ...
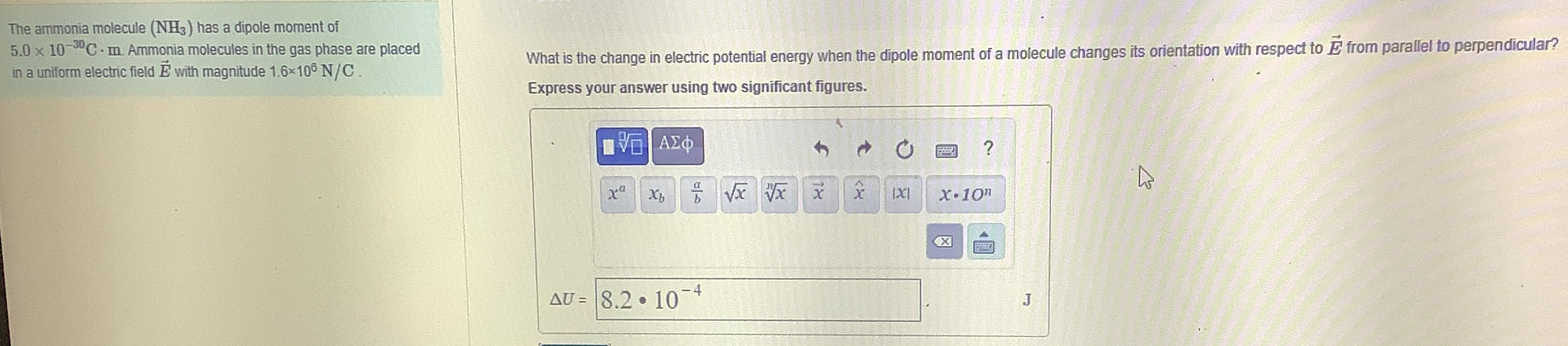
The ammonia molecule
(NH_(3))has a dipole moment of
5.0\times 10^(-30)C*mAmmonia molecules in the gas phase are placed in a uniform electric field
vec(E)with magnitude
1.6\times 10^(6)(N)/(C). What is the change in electric potential energy when the dipole moment of a molecule changes its orientation with respect to
vec(E)from parallel to perpendicular? Express your answer using two significant figures.