Home /
Expert Answers /
Chemistry /
the-average-rate-of-deappearance-of-ozone-in-the-reaction-20-fal-30-al-is-found-to-be-8-83x10-atm-pa804
(Solved): The average rate of deappearance of ozone in the reaction 20,fal --30-al is found to be 8.83x10 atm ...

![Consider the initial rate data given below for the reaction A? 2A.
Rate (Ls) [A]o (mol/L)
6.54
0.75
26.2
1.5
72.7
2.5
What is](https://media.cheggcdn.com/study/2c6/2c6b4746-efb8-47a8-8477-1d95220969db/image)

The average rate of deappearance of ozone in the reaction 20,fal --30-al is found to be 8.83x10 atm over a certain interval of time. What is the rate of appearance of O, during this interval? 8.83×10 atm/s 05.89x10 atm/s 3.44×10¹ m/s O2.00-10¹ atv's 1.32×10 atm/s
Consider the initial rate data given below for the reaction A? 2A. Rate (Ls) [A]o (mol/L) 6.54 0.75 26.2 1.5 72.7 2.5 What is the value of the rate constant? 11.6 O 0.116 0.086 O More data is needed to answer. O None of the Above
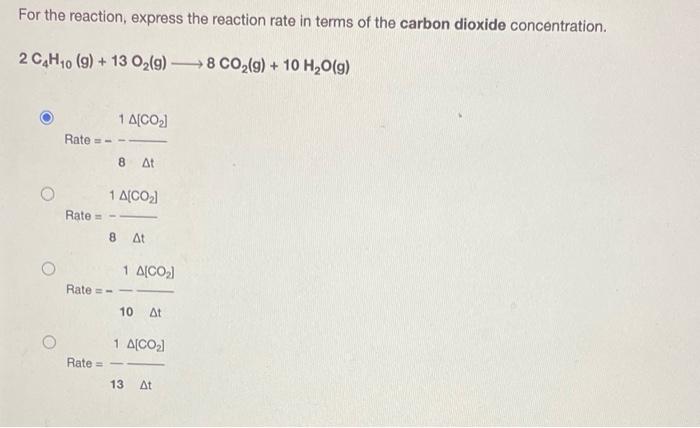
For the reaction, express the reaction rate in terms of the carbon dioxide concentration. 2 C4H10 (9) + 13 O?(g) ?8 CO?(g) + 10 H?O(g) Rate =- Rate= Rate Rate = 1 A[CO?] 8 At 1 A[CO?] 8 At 1 A(CO?) 10 At 1 A[CO?] 13 At
Let the rate of the reaction 2 NH?N? + 3 H? be expressed as -A[NH,/At. Which of the following is the correct expression for the rate of the reaction in terms of A[H?1/At? A[NH?/At 1/2 A[H?/At O ANH J/At=3/2 A[MjJ/At O AINH?J/At 2 A[H?]/At OAINH?J/At-1/3 A[H?/At OA[NH?J/At-2/3 A[H?J/At