Home /
Expert Answers /
Chemistry /
the-compound-ammonium-iodide-nh4i-is-soluble-in-water-write-the-formulas-for-the-ions-that-intera-pa474
(Solved): The compound ammonium iodide, NH4I is soluble in water. Write the formulas for the ions that intera ...

![[Review Topics]
[References]
Use the References to access important values if needed for this question.
How many grams of amm](https://media.cheggcdn.com/study/762/7627e87d-d3e2-4a6a-92b2-d19b53561455/image)
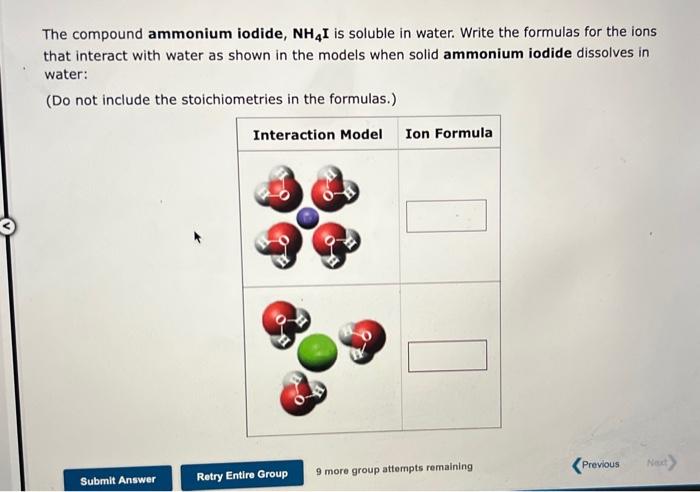
The compound ammonium iodide, NH4I is soluble in water. Write the formulas for the ions that interact with water as shown in the models when solid ammonium iodide dissolves in water: (Do not include the stoichiometries in the formulas.) Interaction Model Ion Formula Previous Next> 9 more group attempts remaining Submit Answer Retry Entire Group
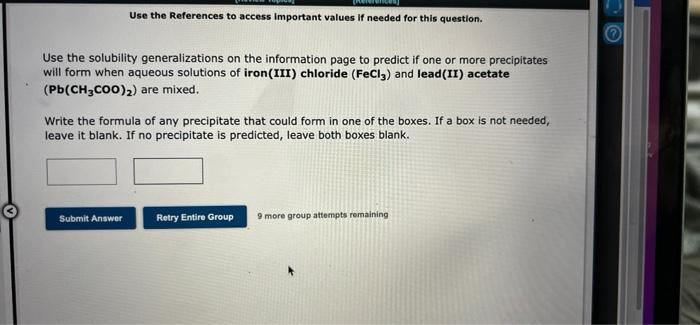
Use the References to access Important values If needed for this question. Use the solubility generalizations on the information page to predict if one or more precipitates will form when aqueous solutions of iron (III) chloride (FeCl3) and lead(II) acetate (Pb(CH3COO)?) are mixed. Write the formula of any precipitate that could form in one of the boxes. If a box is not needed, leave it blank. If no precipitate is predicted, leave both boxes blank. Submit Answer Retry Entire Group 9 more group attempts remaining
Use the References to access important values if needed for this question. How many mL of a 0.144 M aqueous solution of iron (II) chloride, FeCl2, must be taken to obtain 4.11 grams of the salt? mL Submit Answer Retry Entire Group 9 more group attempts remaining
[Review Topics] [References] Use the References to access important values if needed for this question. How many grams of ammonium chloride, NH, CI, must be dissolved to prepare 400. mL of a 0.138 M aqueous solution of the salt? 9 Submit Answer Retry Entire Group 9 more group attempts remaining