Home /
Expert Answers /
Chemistry /
the-decomposition-of-hydrogen-peroxide-h-2-o-2-in-an-aqueous-solution-is-a-reaction-that-can-pa467
(Solved): The decomposition of hydrogen peroxide ( H_(2)O_(2) ) in an aqueous solution is a reaction that can ...
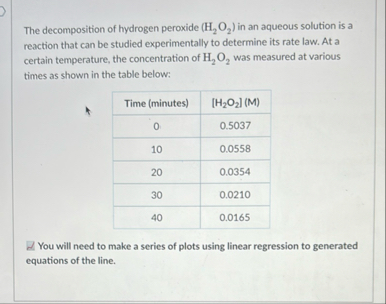
The decomposition of hydrogen peroxide (
H_(2)O_(2)) in an aqueous solution is a reaction that can be studied experimentally to determine its rate law. At a certain temperature, the concentration of
H_(2)O_(2)was measured at various times as shown in the table below: What is the irder if thus reaction? Calculate the rate constant, k, for the reaction. What woukd be the cincentration at 25.0 min