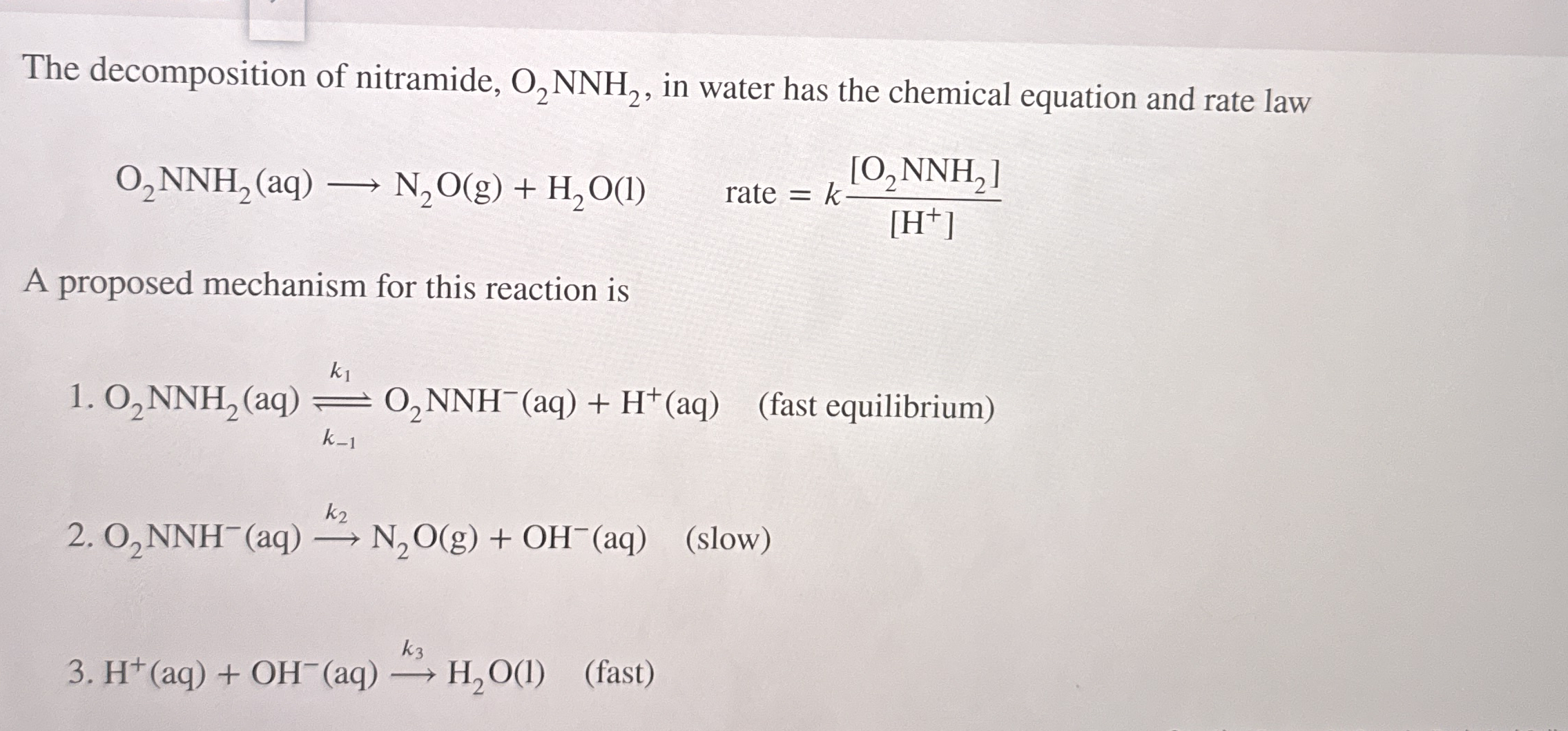Home /
Expert Answers /
Chemistry /
the-decomposition-of-nitramide-o-2-nnh-2-in-water-has-the-chemical-equation-and-rate-law-o-2-n-pa599
(Solved): The decomposition of nitramide, O_(2)NNH_(2), in water has the chemical equation and rate law O_(2)N ...
The decomposition of nitramide,
O_(2)NNH_(2), in water has the chemical equation and rate law
O_(2)NNH_(2)(aq)longrightarrowN_(2)O(g)+H_(2)O(l), rate =k([O_(2)NNH_(2)])/([H^(+)])A proposed mechanism for this reaction is
O_(2)NNH_(2)(aq)?_(k_(-1))^(k_(1))O_(2)NNH^(-)(aq)+H^(+)(aq),(fast equilibrium)
O_(2)NNH^(-)(aq)->k_(2)N_(2)O(g)+OH^(-)(aq) (slow)
H^(+)(aq)+OH^(-)(aq)->k_(3)H_(2)O(1) (fast)