Home /
Expert Answers /
Chemistry /
the-equilibrium-constant-for-the-reaction-sr-s-mg-2-aq-sr-2-aq-mg-s-is-1-90-times-10-29-pa270
(Solved): The equilibrium constant for the reaction Sr(s)+Mg^(2+)(aq)Sr^(2+)(aq)+Mg(s) is 1.90\times 10^(29 ...
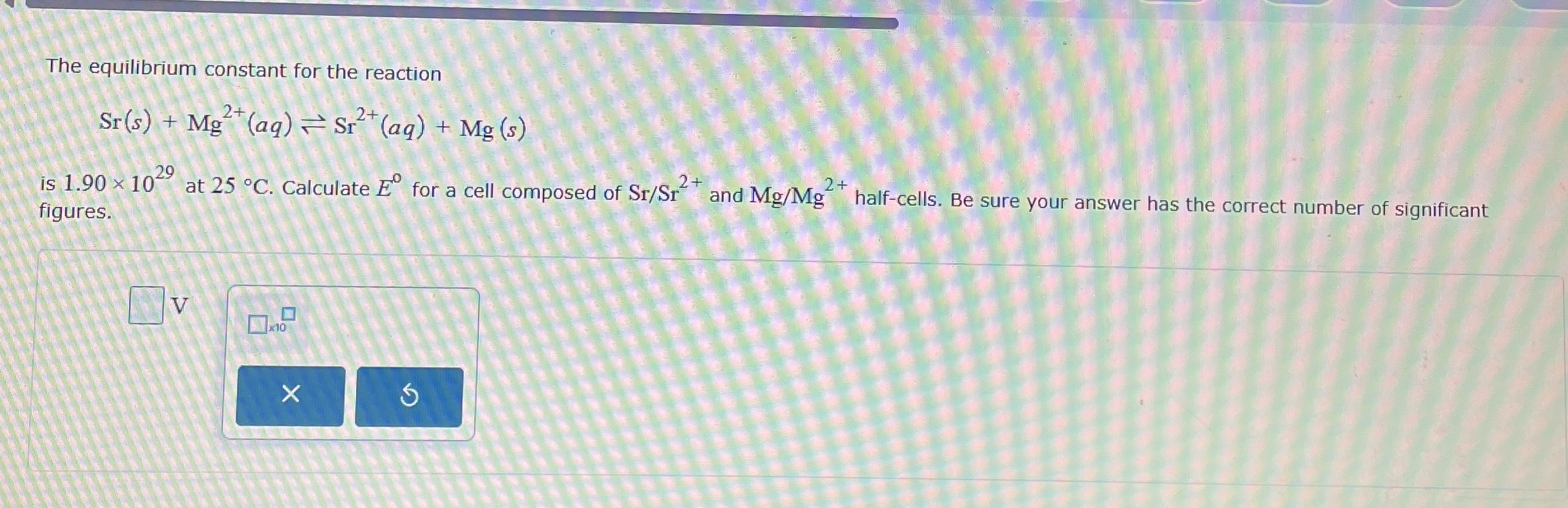
The equilibrium constant for the reaction
Sr(s)+Mg^(2+)(aq)?Sr^(2+)(aq)+Mg(s)is
1.90\times 10^(29)at
25\deg C. Calculate
E^(0)for a cell composed of
S(r)/(S)r^(2+)and
M(g)/(M)g^(2+)half-cells. Be sure your answer has the correct number of significant figures.
?V
?110