Home /
Expert Answers /
Chemistry /
the-equilibrium-constant-in-terms-of-pressures-k-p-for-the-reaction-mathrm-nh-3-m-pa194
(Solved): The equilibrium constant in terms of pressures, \( K_{p} \), for the reaction \[ \mathrm{NH}_{3}(\m ...
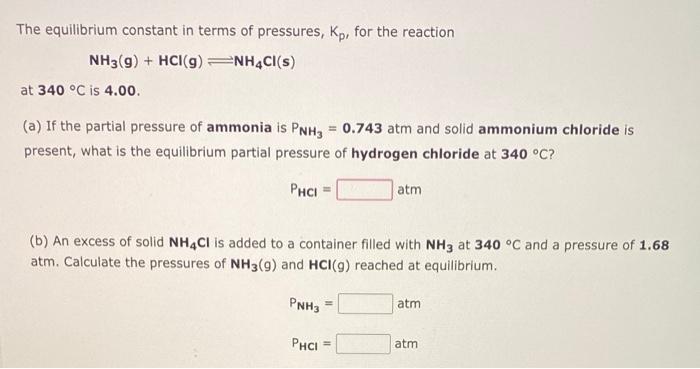
The equilibrium constant in terms of pressures, \( K_{p} \), for the reaction \[ \mathrm{NH}_{3}(\mathbf{g})+\mathrm{HCl}(\mathbf{g}) \rightleftharpoons \mathrm{NH}_{4} \mathrm{Cl}(\mathbf{s}) \] at \( 340^{\circ} \mathrm{C} \) is \( 4.00 \) (a) If the partial pressure of ammonia is \( \mathrm{P}_{\mathrm{NH}_{3}}=\mathbf{0 . 7 4 3} \mathrm{atm} \) and solid ammonium chloride is present, what is the equilibrium partial pressure of hydrogen chloride at \( 340^{\circ} \mathrm{C} \) ? \[ \mathrm{P}_{\mathrm{HCl}}=\mathrm{atm} \] (b) An excess of solid \( \mathbf{N H}_{4} \mathrm{Cl} \) is added to a container filled with \( \mathrm{NH}_{3} \) at \( 340^{\circ} \mathrm{C} \) and a pressure of \( 1.68 \) atm. Calculate the pressures of \( \mathbf{N H}_{3}(g) \) and \( \mathbf{H C l}(g) \) reached at equilibrium. \[ \begin{array}{ll} P_{\mathrm{NH}_{3}}= & \mathrm{atm} \\ \mathrm{P}_{\mathrm{HCl}}= & \text { atm } \end{array} \]