Home /
Expert Answers /
Chemistry /
the-first-ionization-energy-of-the-oxygen-molecule-is-the-energy-required-for-the-following-process-pa891
(Solved): The first ionization energy of the oxygen molecule is the energy required for the following process: ...
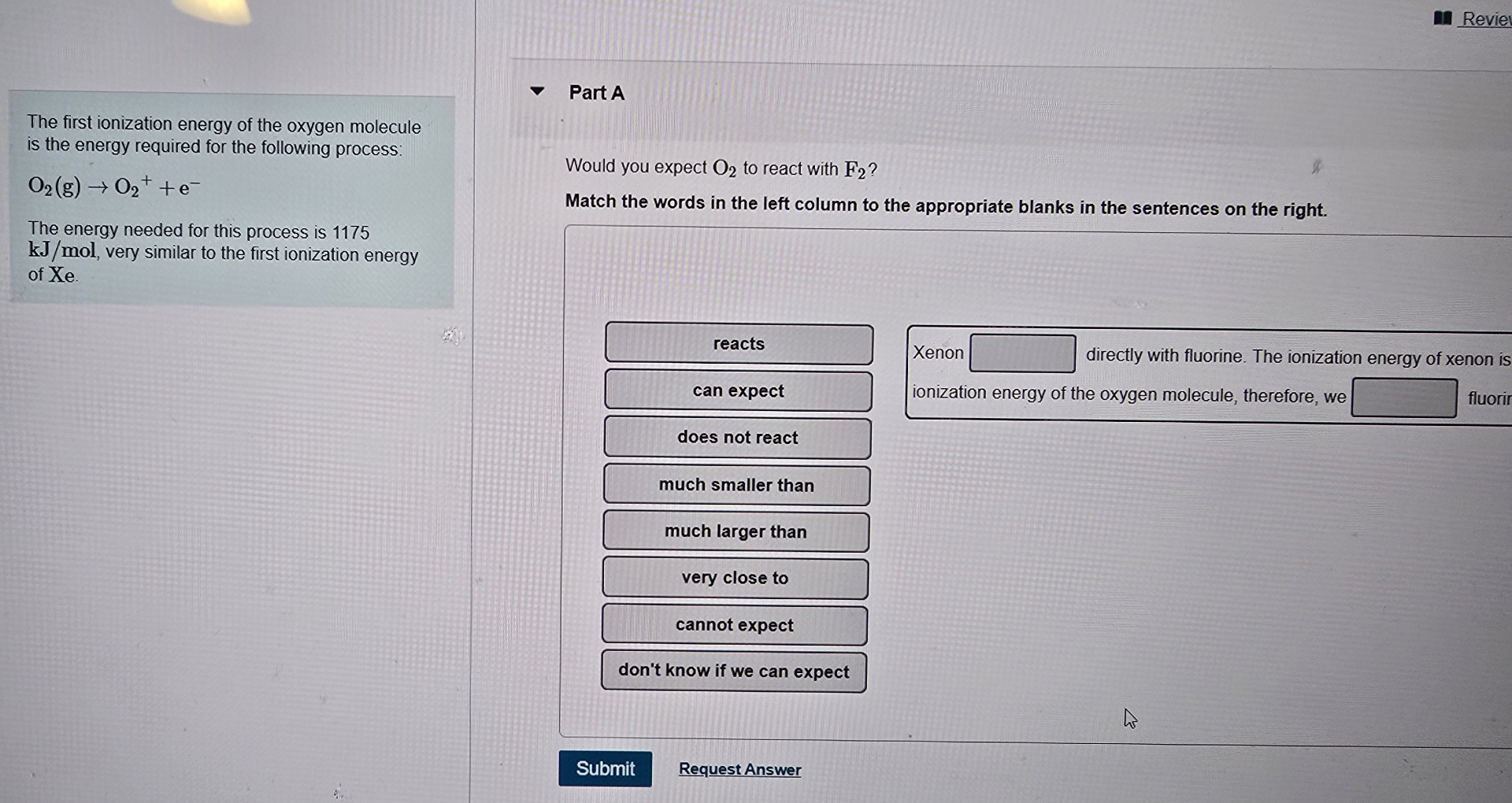
The first ionization energy of the oxygen molecule is the energy required for the following process:
O_(2)(g)->O_(2)^(+)+e^(-)The energy needed for this process is 1175
k(J)/(m)ol, very similar to the first ionization energy of Xe . Part A Would you expect
O_(2)to react with
F_(2)? Match the words in the left column to the appropriate blanks in the sentences on the right. reacts can expect does not react much smaller than much larger than very close to cannot expect don't know if we can expect Xenon ionization energy of the oxygen molecule, therefore, we directly with fluorine. The ionization energy of xenon is