Home /
Expert Answers /
Chemical Engineering /
the-following-isomerization-reaction-occurs-in-the-liquid-phase-a-l-b-l-where-a-and-b-are-misci-pa824
(Solved): The following isomerization reaction occurs in the liquid phase: A(l)B(l) where A and B are misci ...
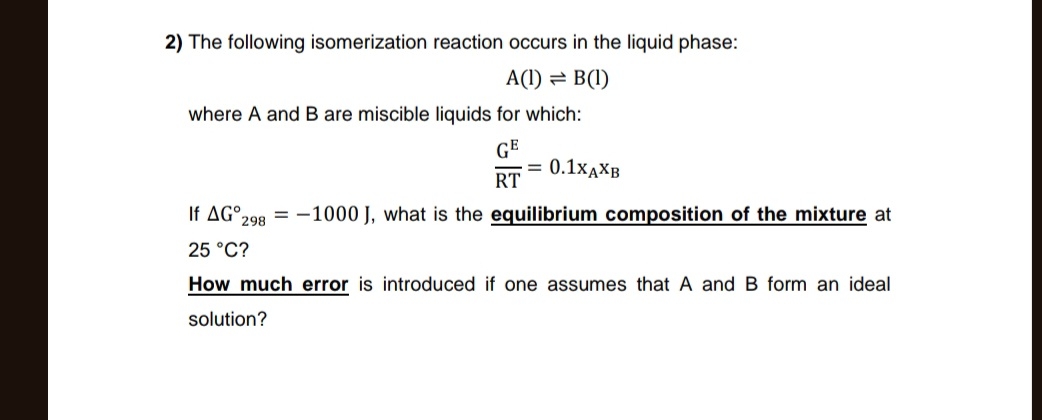
The following isomerization reaction occurs in the liquid phase:
A(l)?B(l)where
Aand
Bare miscible liquids for which:
(G^(E))/(RT)=0.1x_(A)x_(B)If
\Delta G\deg _(298)=-1000J, what is the equilibrium composition of the mixture at
25\deg C? How much error is introduced if one assumes that
Aand
Bform an ideal solution?