Home /
Expert Answers /
Chemistry /
the-lab-and-the-data-have-been-done-can-you-please-show-how-the-calculations-are-done-for-futher-he-pa320
(Solved): the lab and the data have been done, can you please show how the calculations are done for futher he ...
the lab and the data have been done, can you please show how the calculations are done for futher help?
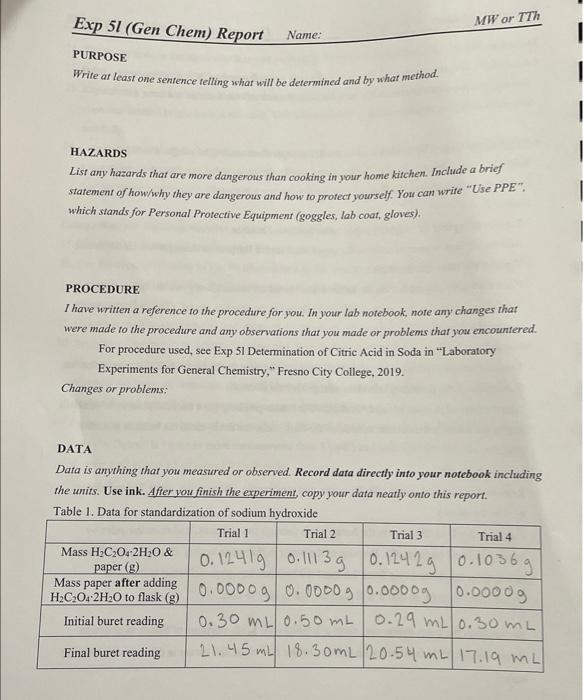





PURPOSE Write at least one sentence felling what will be determined and by what method. HAZARDS List any hazards that are more dangenous than cooking in your home kitchen. Include a brief staiement of how/why they are dangerous and how to profect yourself. You can write "Use PPE". which stands for Personal Protective Equipment (goggles, lab coat, gloves). PROCEDURE I have written a reference to the procedure for you. In your lab notebook, note any changes that were made to the procedure and any observations that you made or problems that you encountered. For procedure used, see Exp 51 Determination of Citric Acid in Soda in "Laboratory Experiments for General Chemistry," Fresno City College, 2019. Changes or problems: DATA Data is anything that you measured or obsenved. Record data directly into your notebook including the units. Use ink. After you finish the experiment, copy your data neatly onto this report. Table 1. Data for standardization of sodium hudroxide
CALCULATIONS Show one example of each calculation, including units. Thave labeled the calculations you should show for this experiment. Work out your calculations in your notebook first and then copy them neatly here. Standardization of sodium hydroxide Mass of oxalic acid dihydrate added to flask: Moles of \( \mathrm{NaOH} \) using this path: \( \mathrm{g} \mathrm{H}_{2} \mathrm{C}_{2} \mathrm{O}_{4} \cdot 2 \mathrm{H}_{2} \mathrm{O} \rightarrow \) mole \( \mathrm{H}_{2} \mathrm{C}_{2} \mathrm{O}_{4} \cdot 2 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{mol} \mathrm{NaOH} \) Net volume of \( \mathrm{NaOH} \) used (L): \( \mathrm{NaOH} \) molarity: Grubbs Test: Determination of citric acid in soda Net volume of \( \mathrm{NaOH} \) used (L): Moles of citric acid using this path: \( \mathrm{L} \mathrm{NaOH} \rightarrow \mathrm{mol} \mathrm{NaOH} \rightarrow \mathrm{mol} \mathrm{H}_{3} \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{O}_{7} \) Molarity of citric acid in "6 Down" soda: Average molarity of citric acid in \( 7 \mathrm{Up} \otimes \) soda*: 60
* " 6 Down" is about 10 times more concentrated in citric acid than 7 Up® so to calculate the Grams of citric acid in one 12-oz (355-mL) can of \( 7 \mathrm{Up} \mathbb{\mathrm { P }} \) :