(Solved): The nitration of aromatic compounds is a highly exothermic reaction thatIt generally uses catalysts ...
The nitration of aromatic compounds is a highly exothermic reaction that
It generally uses catalysts that tend to be corrosive. A reaction
The less corrosive N2O5 is used as a nitrating agent as illustrated
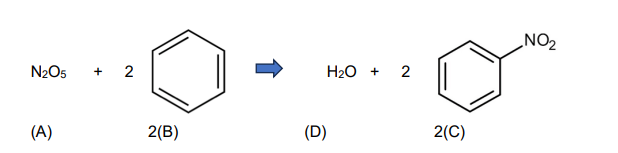
If this reaction takes place in a liquid-phase adiabatic CSTR, 35% conversion of N2O5
How would it be determined whether this reactor, under these reaction conditions,
Does it have other steady states? Explain the procedure, it does not require
Do the math. You can use diagrams to be clearer
Additional data:
The rate of reaction is first-order with respect to A and second-order with respect to B. ?????????(303????) = ?370,1 ????????/???????????? ???? 0 = 303 ???? ???????????? = 84,5 ????/(???????????? ? ????) ???????? 0 = 10 ????????????/???????????? ???????????? = 137 ????/(???????????? ? ????) ???????? 0 = 30 ????????????/???????????? ???????????? = 170????/(???????????? ? ????) ???? = 1000 ????/???????????? ???????????? = 75 ????/(???????????? ? ????) ???????? 0 = 0,01 ????????????/L