Home /
Expert Answers /
Chemistry /
the-normal-boiling-point-of-water-is-100-deg-c-at-which-temperature-does-a-vapour-bubble-of-radius-pa699
(Solved): The normal boiling point of water is 100\deg C. At which temperature does a vapour bubble of radius ...
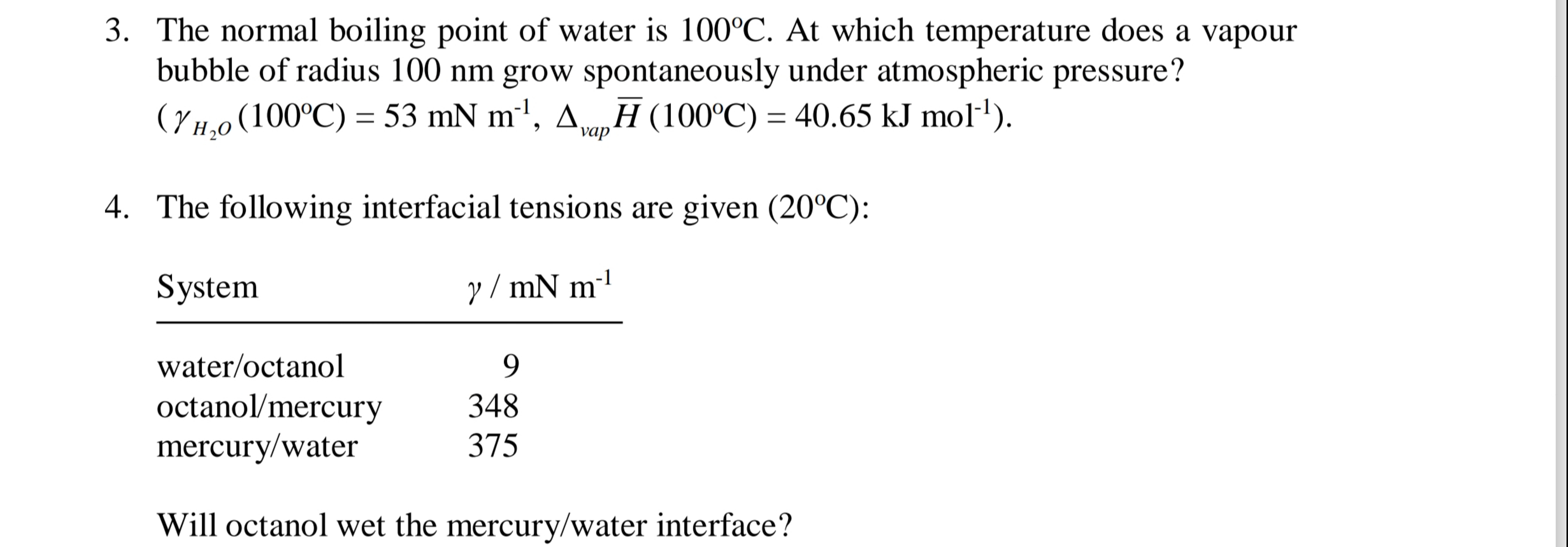
The normal boiling point of water is
100\deg C. At which temperature does a vapour bubble of radius 100 nm grow spontaneously under atmospheric pressure?
(\gamma _(H_(2)O)(100\deg C)=53mN(m)^(-1),\Delta _(vap )(()/(bar) (H))(100\deg C)=40.65(kJ)mol^(-1))The following interfacial tensions are given
(20\deg C): Will octanol wet the mercury/water interface?