Home /
Expert Answers /
Chemistry /
the-reaction-for-the-formation-of-phosgene-from-carbon-monoxide-and-chlorine-mathrm-co-mathrm-pa246
(Solved): The reaction for the formation of phosgene from carbon monoxide and chlorine \[ \mathrm{CO}+\mathrm ...

The reaction for the formation of phosgene from carbon monoxide and chlorine \[ \mathrm{CO}+\mathrm{Cl}_{2} \rightarrow \mathrm{COCl}_{2} \] is first order in \( \mathrm{CO} \) and second order overall. Complete the rate law for this reaction in the box below. Use the form \( k[\mathrm{~A}]^{m}[\mathrm{~B}]^{n} \), where ' 1 ' is understood for \( m \) or \( n \) and concentrations taken to the zero power do not appear. Don't enter 1 for \( m \) or \( n \). Rate \( = \)
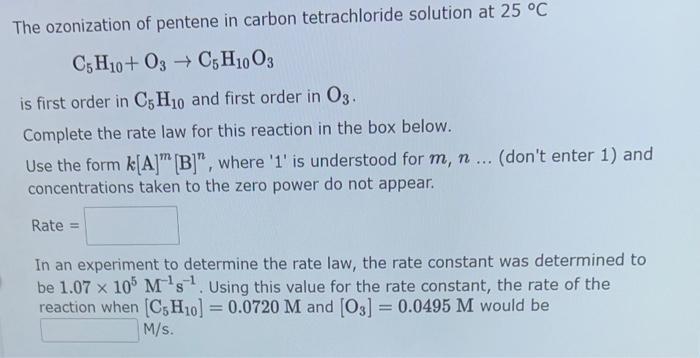
The ozonization of pentene in carbon tetrachloride solution at \( 25^{\circ} \mathrm{C} \) \[ \mathrm{C}_{5} \mathrm{H}_{10}+\mathrm{O}_{3} \rightarrow \mathrm{C}_{5} \mathrm{H}_{10} \mathrm{O}_{3} \] is first order in \( \mathrm{C}_{5} \mathrm{H}_{10} \) and first order in \( \mathrm{O}_{3} \). Complete the rate law for this reaction in the box below. Use the form \( k[\mathrm{~A}]^{m}[\mathrm{~B}]^{n} \), where ' 1 ' is understood for \( m, n \ldots \) (don't enter 1 ) and concentrations taken to the zero power do not appear. Rate \( = \) In an experiment to determine the rate law, the rate constant was determined to be \( 1.07 \times 10^{5} \mathrm{M}^{-1} \mathrm{~s}^{-1} \). Using this value for the rate constant, the rate of the reaction when \( \left[\mathrm{C}_{5} \mathrm{H}_{10}\right]=0.0720 \mathrm{M} \) and \( \left[\mathrm{O}_{3}\right]=0.0495 \mathrm{M} \) would be \( \mathrm{m} / \mathrm{s} \).
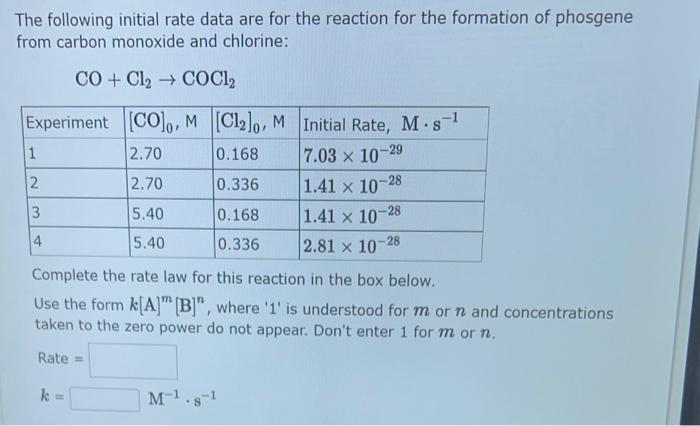
The following initial rate data are for the reaction for the formation of phosgene from carbon monoxide and chlorine: \[ \mathrm{CO}+\mathrm{Cl}_{2} \rightarrow \mathrm{COCl}_{2} \] Complete the rate law for this reaction in the box below. Use the form \( k[\mathrm{~A}]^{m}[\mathrm{~B}]^{n} \), where ' 1 ' is understood for \( m \) or \( n \) and concentrations taken to the zero power do not appear. Don't enter 1 for \( m \) or \( n \).
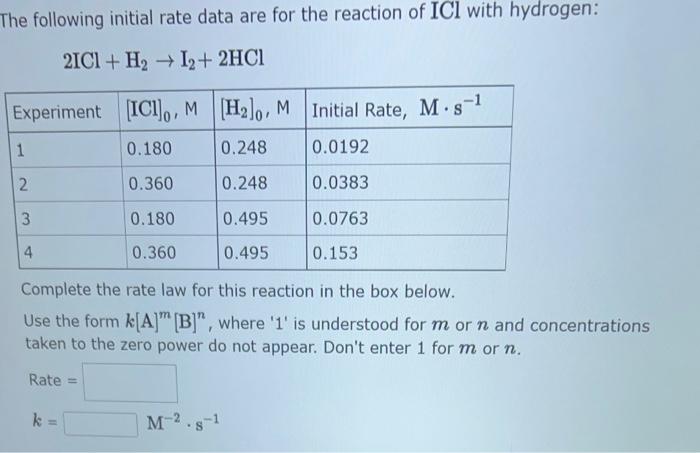
The following initial rate data are for the reaction of ICl with hydrogen: \[ 2 \mathrm{ICl}+\mathrm{H}_{2} \rightarrow \mathrm{I}_{2}+2 \mathrm{HCl} \] Complete the rate law for this reaction in the box below. Use the form \( k[\mathrm{~A}]^{m}[\mathrm{~B}]^{n} \), where ' 1 ' is understood for \( m \) or \( n \) and concentrations taken to the zero power do not appear. Don't enter 1 for \( m \) or \( n \).