Home /
Expert Answers /
Chemistry /
the-reaction-to-produce-ethylene-oxide-is-as-follows-c2h4-1-2o2c2h4o-the-reactio-pa344
(Solved): The reaction to produce ethylene oxide is as follows; C2H4+1/2O2C2H4O The reactio ...
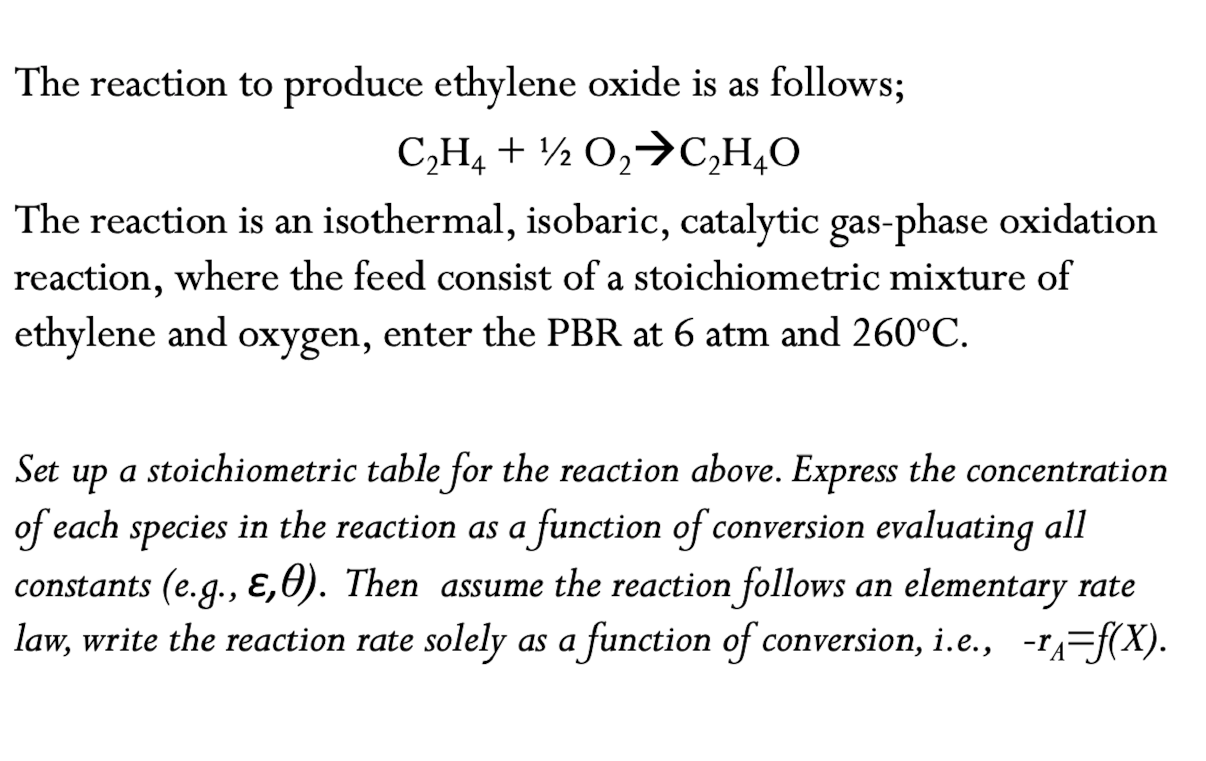
The reaction to produce ethylene oxide is as follows; The reaction is an isothermal, isobaric, catalytic gas-phase oxidation reaction, where the feed consist of a stoichiometric mixture of ethylene and oxygen, enter the PBR at and . Set up a stoichiometric table for the reaction above. Express the concentration of each species in the reaction as a function of conversion evaluating all constants (e.g., . Then assume the reaction follows an elementary rate law, write the reaction rate solely as a function of conversion, i.e., .