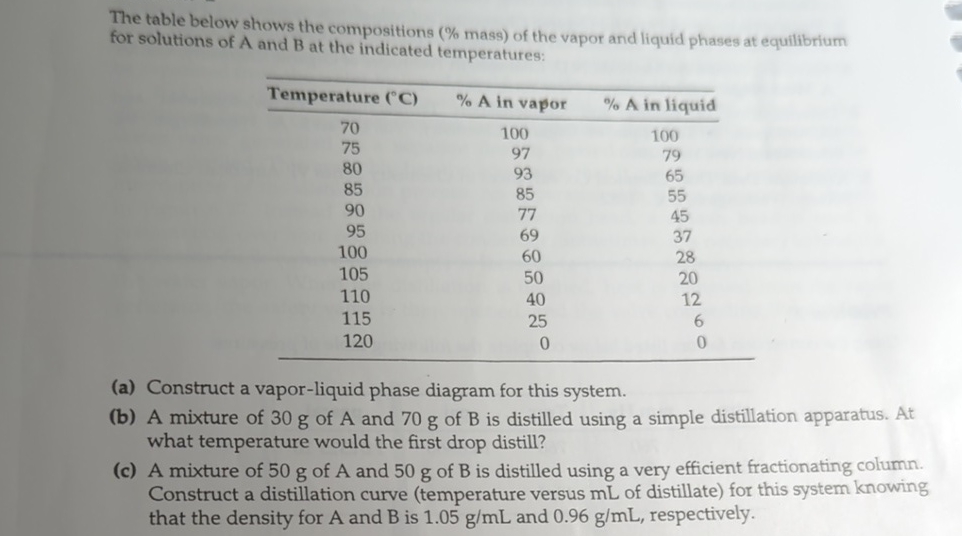
(Solved): The table below shows the compositions (% mass) of the vapor and liquid phases at equilibrium for so ...
The table below shows the compositions (% mass) of the vapor and liquid phases at equilibrium for solutions of A and B at the indicated temperatures: \table[[Temperature (
\deg C),
%Ain vapor,
%Ain liquid],[70,100,100],[75,97,79],[80,93,65],[85,85,55],[90,77,45],[95,69,37],[100,60,28],[105,50,20],[110,40,12],[115,25,6],[120,0,0]] (a) Construct a vapor-liquid phase diagram for this system. (b) A mixture of 30 g of A and 70 g of B is distilled using a simple distillation apparatus. At what temperature would the first drop distill? (c) A mixture of 50 g of A and 50 g of B is distilled using a very efficient fractionating column. Construct a distillation curve (temperature versus mL of distillate) for this system knowing that the density for
Aand
Bis
1.05(g)/(m)Land
0.96(g)/(m)L, respectively. Please explain part c in detail.