(Solved): The total driving force, called the electrochemical force, is determined by looking at both forces ...
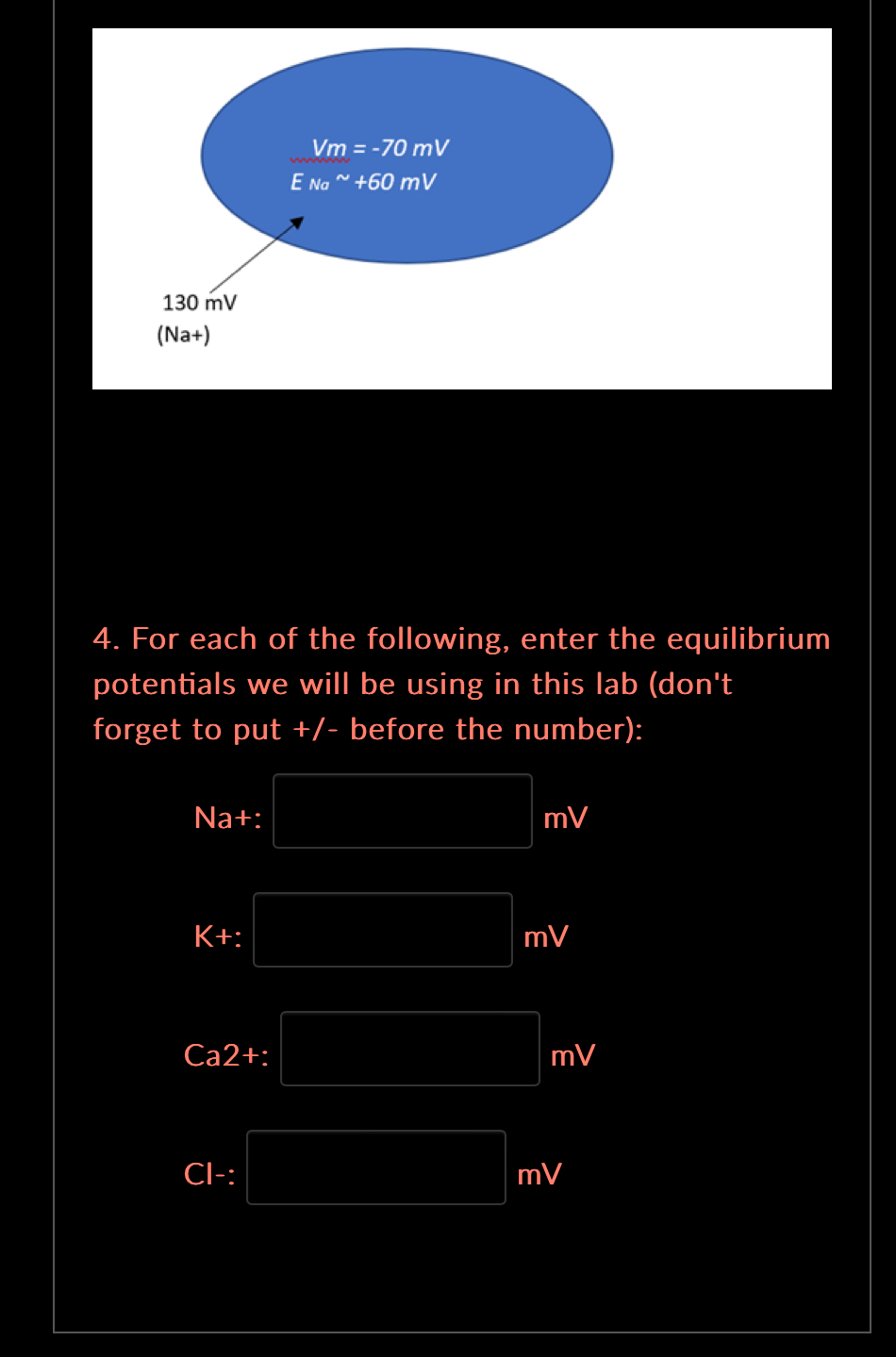
The total driving force, called the electrochemical force, is determined by looking at both forces and summing them together and whichever is stronger determines the direction and magnitude of the resulting force. If both forces, go in the same direction then the resulting force will go in that direction and the magnitude will be determined by how strong both forces are when combined. If the two forces don’t both go in the same direction, then how do we determine which way and what the magnitude will be? First, you need to know the ion in question’s equilibrium potential. An ion’s equilibrium potential is the hypothetical value of when the chemical and electrical gradients are exactly equal and opposite for that ion. This value is determined by the size and direction of the ion’s concentration gradient and its charge. The ion’s equilibrium potential is calculated using the Nernst Equation. In this lab you will be using the following equilibrium potentials: ENa+ is ~ +60 mV EK+ is ~ -94 mV ECa2+ is ~ +135 mV ECl- is ~ -88 mV What is the direction and magnitude of the electrochemical force acting on these ions? How do we figure this out? First - compare the ion’s equilibrium potential to the membrane potential (-70 mV). Are these two values the same? If the answer is yes, then the ion is at dynamic equilibrium and there is no net force acting on it. Second - if the answer is no, which it is for both ions, then you will have to compare the membrane potential to each of the equilibrium potentials to calculate the direction and magnitude of the force acting on each ion. For example: Na+ will move until the membrane potential, -70 mV, becomes +60 mV (Na+ equilibrium potential). The answer is that Na+ would move into the cell because as each Na+ moves in, the membrane potential becomes less negative – moving towards the +60. So now we have the direction (inward to the ICF), what about the magnitude? Simple! How many millivolts difference is there between -70 mV and +60 mV? The answer is 130 mV – note that force cannot be negative. Magnitude Force.png 4. For each of the following, enter the equilibrium potentials we will be using in this lab (don't forget to put +/- before the number): Na+: mV K+: mV Ca2+: mV Cl-: mV For each of the following, enter the equilibrium potentials we will be using in this lab (don't forget to put +/- before the number):