Home /
Expert Answers /
Chemistry /
two-reactions-and-their-equilibrium-constants-are-given-a-2b-2c-k-2-61-2c-d-k-0-214-ca-pa248
(Solved): Two reactions and their equilibrium constants are given. A + 2B 2C K = 2.61 2C D K = 0.214 Ca ...

Two reactions and their equilibrium constants are given. A + 2B 2C K? = 2.61 2C D K? = 0.214 Calculate the value of the equilibrium constant for the reaction D = A + 2B. K =
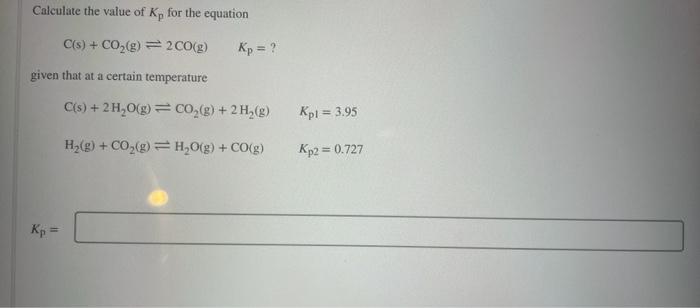
Calculate the value of K, for the equation C(s) + CO?(g) = 2 CO(g) given that at a certain temperature Kp = Kp = ? C(s) + 2H?O(g) = CO?(g) + 2 H?(g) H?(g) + CO?(g) = H?O(g) + CO(g) Kpl = 3.95 Kp2 = 0.727