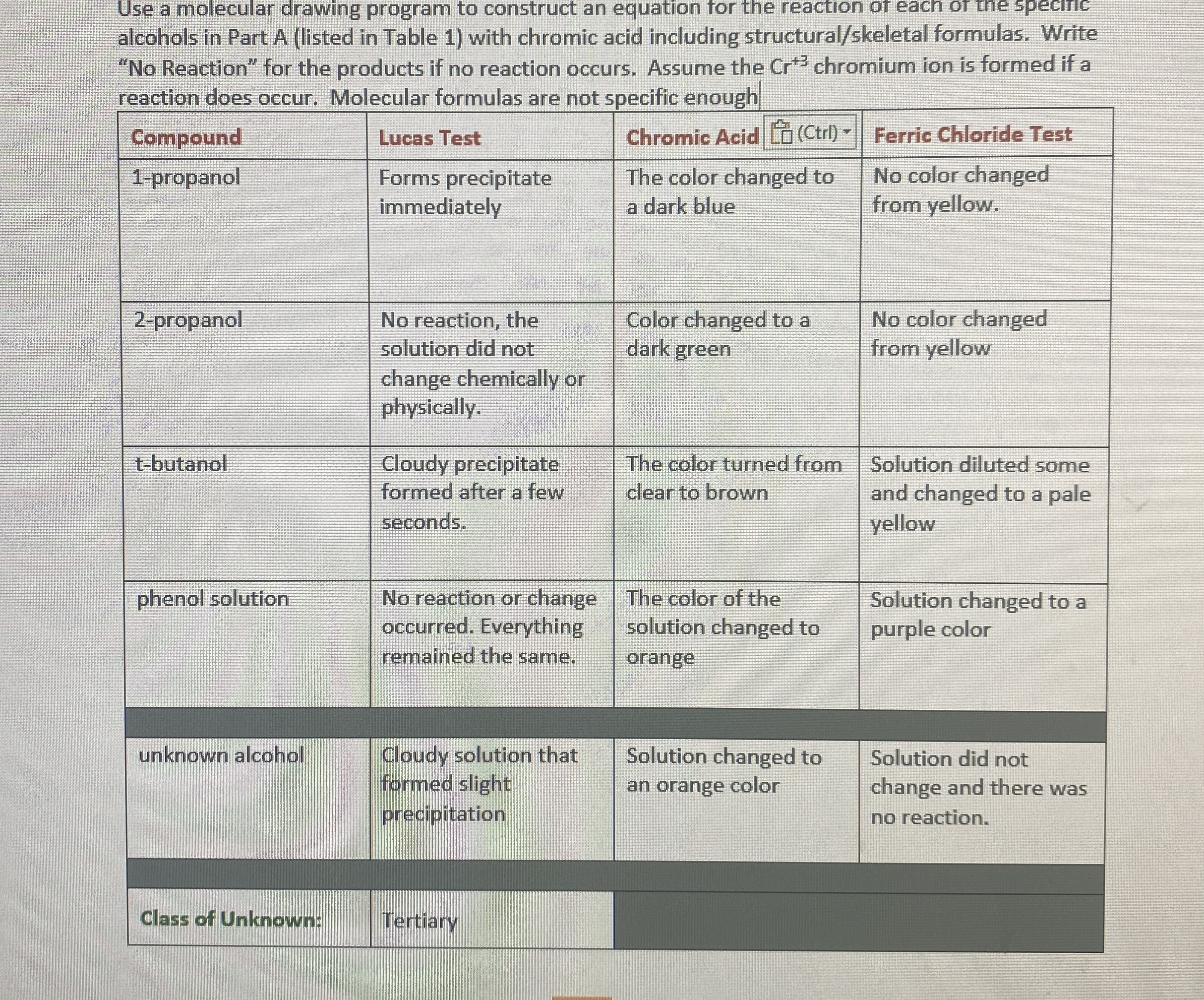
(Solved): Use a molecular drawing program to construct an equation for the reaction of each of the speciric al ...
Use a molecular drawing program to construct an equation for the reaction of each of the speciric alcohols in Part A (listed in Table 1) with chromic acid including structural/skeletal formulas. Write "No Reaction" for the products if no reaction occurs. Assume the
Cr^(+3)chromium ion is formed if a reaction does occur. Molecular formulas are not specific enough \table[[Compound,Lucas Test,Chromic Acid
[[ Laltr) ],Ferric Chloride Test],[1-propanol,\table[[Forms precipitate],[immediately]],\table[[The color changed to],[a dark blue]],\table[[No color changed],[from yellow.]]],[2-propanol,\table[[No reaction, the],[solution did not],[change chemically or],[physically.]],\table[[Color changed to a],[dark green]],\table[[No color changed],[from yellow]]],[t-butanol,\table[[Cloudy precipitate],[formed after a few],[seconds.]],\table[[The color turned from],[clear to brown]],\table[[Solution diluted some],[and changed to a pale],[yellow]]],[phenol solution,\table[[No reaction or change],[occurred. Everything],[remained the same.]],\table[[The color of the],[solution changed to],[orange]],\table[[Solution changed to a],[purple color]]],[Class of Unknown:,\table[[Cloudy solution that]],\table[[Solution changed to],[an orange color]],\table[[Solution did not],[change and there was],[no reaction.]]]]