Home /
Expert Answers /
Chemistry /
use-standard-reduction-potentials-to-calculate-the-equilibrium-constant-for-the-reaction-2h-aq-2-pa862
(Solved): Use standard reduction potentials to calculate the equilibrium constant for the reaction: 2H+(aq)+2 ...
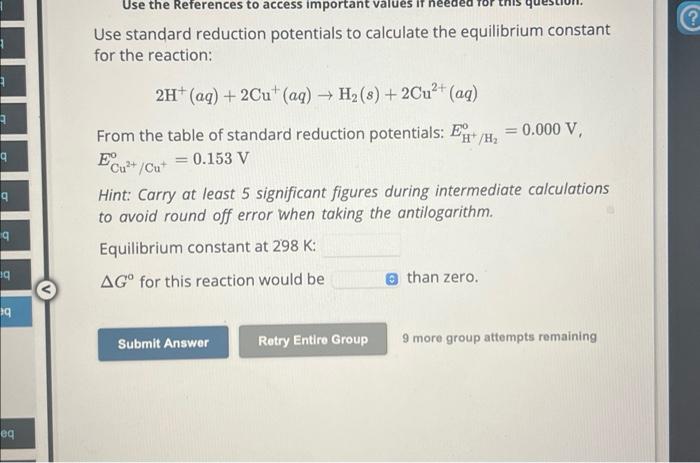
Use standard reduction potentials to calculate the equilibrium constant for the reaction: From the table of standard reduction potentials: , Hint: Carry at least 5 significant figures during intermediate calculations to avoid round off error when taking the antilogarithm. Equilibrium constant at : for this reaction would be than zero. 9 more group attempts remaining