Home /
Expert Answers /
Chemical Engineering /
use-the-given-formulas-to-solve-a-the-following-multiple-reactions-involving-methane-gas-ch-4-t-pa307
(Solved): use the given formulas to solve(a) The following multiple reactions involving methane gas (CH_(4)) t ...
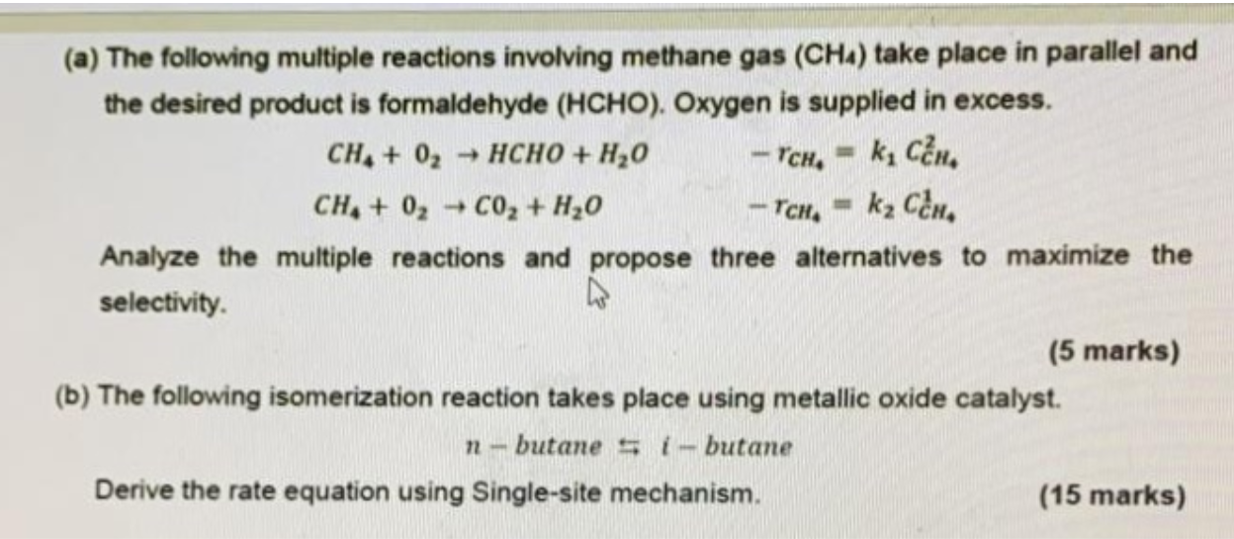
use the given formulas to solve(a) The following multiple reactions involving methane gas (CH_(4)) take place in parallel and
the desired product is formaldehyde ( HCHO ). Oxygen is supplied in excess.
CH_(4)+O_(2)->HCHO+H_(2)O,-r_(CH_(4))=k_(1)C_(CH)^(2)
CH_(4)+O_(2)->CO_(2)+H_(2)O,-r_(CH_(4))=k_(2)C_(CH)^(1)
Analyze the multiple reactions and propose three alternatives to maximize the
selectivity.
(b) The following isomerization reaction takes place using metallic oxide catalyst.
n-butane ?i-butane
Derive the rate equation using Single-site mechanism.